Applying Health Literacy to Informed Consents for Research:
Quick Tips for Clearly Explaining the Requirements
Adapted from original material created by: Christopher R. Trudeau, JD

Creating consent materials that people can understand involves not only thinking about the content needed, but also how that content will be worded, ordered, and presented. Consent form content is always important, yet due to recent regulatory changes that emphasize clarity, consent forms will be reviewed more closely by Institutional Review Boards/Ethics Committees (IRB/ECs), government regulators, and, sometimes, even in courts.
Using health literacy best practices, we can do more with consent forms than simply ensuring regulatory compliance.
Find out how to:
- Prepare to Create a Clear Informed Consent Form
- Draft the Content in Plain Language
Note: Unless quoting specific regulations that refer to “subjects,” we will use the preferred term “participant” to describe individuals who decide to join a research study.
Preparing to Create a Clear Informed Consent Form
Creating any health communication takes planning and preparation.
This section outlines the steps needed to create consent forms for research that not only comply with regulations but also present the content in ways that participants are more likely to understand. Many of these steps are, of course, applicable to the creation of all consent forms in any country, although country-specific regulations would need to be considered. Here we will use the US regulatory framework by way of example.
- Step One – Address the Three Pillars of Consent: Purpose, Audience, and Process
- Step Two – Determine the Legal Requirements for Consent
- Step Three – Create a Preliminary Outline of Needed Requirements
- Step Four – Plan Your Design Strategy
Step One – Address the Three Pillars of Consent: Purpose, Audience, and Process
Consent forms are not transactional documents like other contracts – they are tools to help facilitate an informed choice of whether or not to join a study. At Step One you must determine Purpose, Audience and Process.
Purpose helps keep you focused when creating a consent form – it is one of the pillars that help you decide what to include and how to explain the information clearly.
Purposes of consent for research:
- Describe the study so that participants are aware of what to expect and can make more autonomous, informed decisions about whether they (or their children) should participate
- Provide a framework to support meaningful consent discussions
- Comply with regulatory requirements
- Insulate researchers, institutions, and sponsors from liability.
One study found that “factors of the consent discussion that were associated with a decreased likelihood of enrollment included . . . the presentation of complex information in a limited amount of time.” [Hallinan, et al, 2016.] https://www.thehastingscenter.org/irb_article/barriers-to-change-in-the-informed-consent-process-a-systematic-literature-review/
Consent forms provide information to
- Potential and enrolled participants – who are seeking clear information to help them decide whether to join and to guide them once they decide to join;
- IRB reviewers – who not only review for regulatory compliance but also advocate for ethical treatment of and protect participants;
- Sponsors & funding agencies – who are increasingly concerned with issues of health literacy and participant understanding due to organizational policy and changing regulations;
- Investigators and research staff – who often are tasked with discussing and obtaining consent from study participants; and
- The general public – especially now that many consent forms are available on clinicaltrials.gov.
Defining all of your consent purposes during the planning process can guide you in making key choices about what to include, where you will include it, and how you will clearly convey the message so that people are able to make informed, autonomous decisions about whether to take part in a research study.
For example, a participant experiencing something new, like a rash, can look back at the consent form to see if it might be a known risk of the study intervention.
Before you start writing the consent form, consider the audiences. Clearly, potential and enrolled study participants are your primary audience. But, as noted above, many others see and can be informed by your consent form, including family members of the participant.
When thinking about your primary audience – study participants – think about what you know about them. Are they young adults? Do most come from a particular cultural background? Are there other medical or social factors that may impact the group? The underlying idea here is that understanding the needs of your study population and addressing possible health literacy challenges will serve all audiences better. You can learn more about cultural considerations in clinical research here
Another, often overlooked, audience for consent forms are research staff. Clear consent forms, with explanations of legal and medical terms, will help research staff by ensuring consistency in educating participants about the study and in obtaining their informed consent.
Involve people from your study population as you develop the study protocol and the consent form to help ensure that you are meeting their informational needs and designing materials for a study that people will want to join.
Consent is more than just a form – it is a process. The consent process begins the moment you start soliciting people to be in the study and ends only after you will no longer use the participant’s information or biospecimens for further research. Thus, it is important to think about this early and create a plan for consenting participants that considers the following:
- How will potential participants be educated about the study?
- When will they be educated about the study?
- When will they receive the consent form?
- How will they receive the consent form (electronically or in print)?
- How long will they have to read and consider the form?
- Who will be there to guide them through the consent process?
- To whom should they ask questions about the study?
- What additional information or materials might help them make an informed decision?
- How can I encourage and support them to ask questions?
- When will they be expected to sign the consent form?
In all cases, as you plan the consent process, be sure that it includes (1) a plan for participant education and (2) includes some opportunity for one-on-one interaction with a member of the study team to answer questions and facilitate understanding.
The best way to do facilitate understanding is by using a technique called teach-back. This involves participants demonstrating knowledge by “teaching” what they learn back to the person that explained it. For more on teach-back click here.
Step Two – Determine the Legal Requirements for Consent
The second step is to determine what is legally required to be included in your consent form. Once you figure out those “must haves,” you can then better consider how to organize and explain those concepts in the form
To better facilitate informed consent decision-making, the US federal regulations (Revised Common Rule) now require that consent forms “begin with a concise and focused presentation of the key information that is most likely to assist a prospective subject or legally authorized representative in understanding the reasons why one might or might not want to participate in the research.” ([46.116(a)(5)(i)]) This requirement will aid in focusing consent forms and processes to the goal of assisting a participant in their decision making.
Facilitating participant understanding is the goal of the informed consent documents and processes in all settings, in all languages, and in all countries; local regulations will apply and may be less explicit than the US federal regulations. Nevertheless, the goal of informed consent is comprehension.
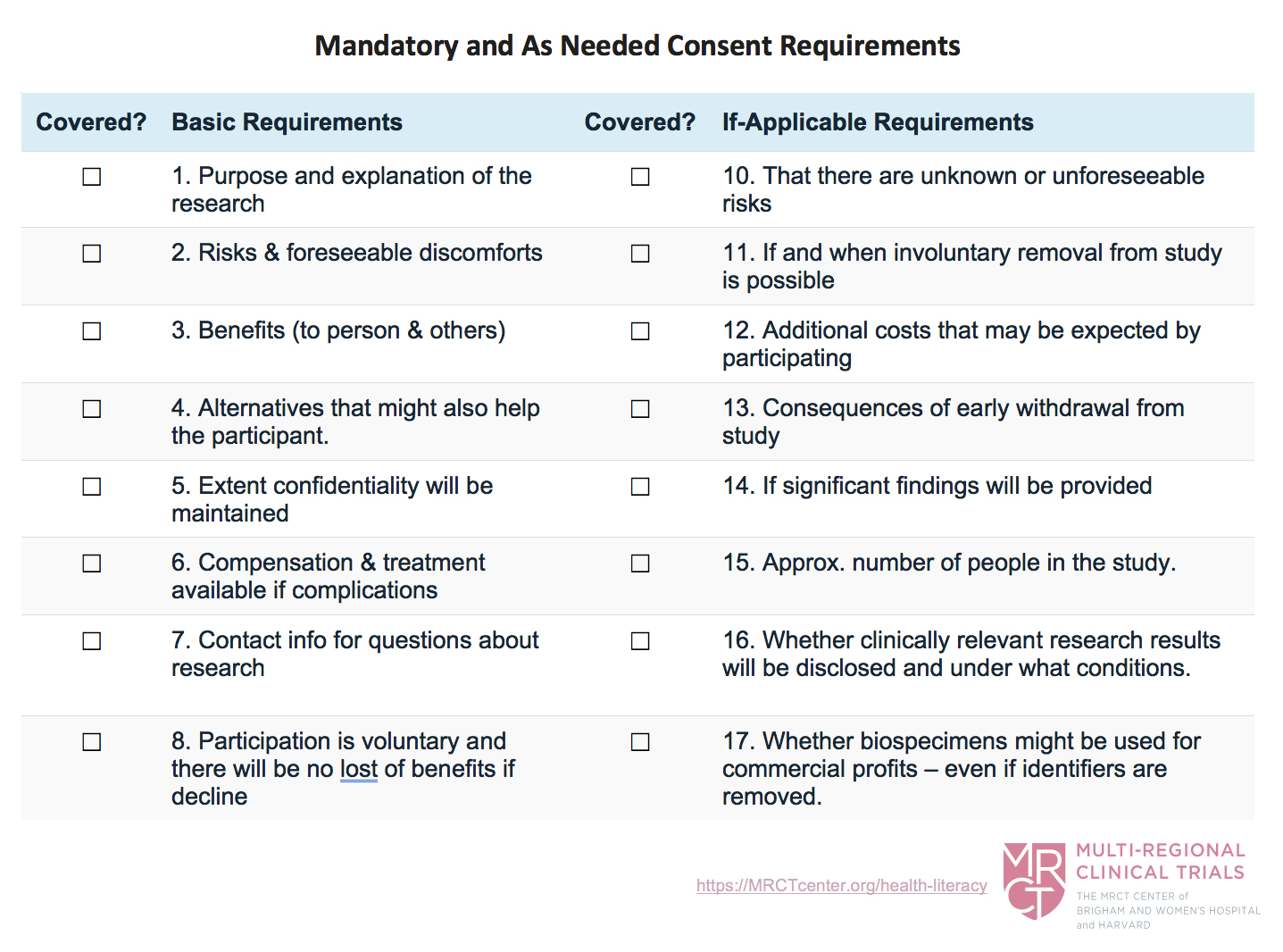
When considering the consent requirements, it helps to keep notes on what US requirements must be included in the consent form. This will help you plan and organize the content in your consent form, and it will help ensure that you do not forget something you planned on including.
Use the following chart which is based on the US requirements to help you keep track of the elements that must be included in your consent forms. This list can be adapted for any country. Use it before writing and when checking drafts.
The next step is to determine if there are any state-specific or industry-specific requirements that relate to your study. While some states and industries have no such laws, other states do. Plus, laws are constantly changing, so research these before beginning to write the consent form. The best place to start is by checking with compliance or regulatory oversight personnel – e.g. IRBs, CTSAs, Offices of Research Protections, Industry Regulators, etc.
In addition, for multi-regional clinical trials, considering the international legal and regulatory requirements is essential. The International Compilation of Human Research Standards – PDF is a listing of over 1,000 laws, regulations, and guidelines on human research participant protections in 131 countries and from many international organizations. These standards are maintained and updated annually by the US Office of Human Research Protection (OHRP).
Step Three – Create an Outline of Needed Requirements
After researching the legally required content for the consent form, Step Three is to create an outline of the content to include. Except for the addition of key information at the beginning of the consent form, you do not have to follow the exact order of requirements as listed in the federal regulations.
For US federally funded trials, the Revised Common Rule now mandates that consent forms “begin with a concise and focused presentation of the key information.” Since this is a new requirement, deciding what to include is not always easy nor need it be consistent from trial to trial. It will vary based on your purpose and audience.
The Revised Common Rule states that the following things may be key information in many studies:
- That consent is for research and participation is voluntary
- The purpose, duration, and procedures of the study
- Any reasonably foreseeable risks or discomforts of joining the study
- Any alternatives to the study that might be advantageous to the participant
- Any benefits to the participants or others
While these five things are listed, you should be flexible in determining what amounts to key information for your specific study. In an appendix to its guidance, SACHRP (the Secretary’s Advisory Committee on Human Research Protection) created the following list of questions to help you determine what might be key information for your study:
- What are the main reasons a subject will want to join this study?
- What are the main reasons a subject will not want to join this study?
- What is the research the question the study is trying to answer? Why is it relevant to the subject?
- What aspects of research participation or this particular study are likely to be unfamiliar to a prospective subject, diverge from a subject’s expectations, or require special attention?
- What information about the subject is being collected as part of this research?
- What are the types of activities that subjects will do in the research?
- What impact will participating in this research have on the subject outside of the research? For example, will it reduce options for standard treatments?
- How will the subjects’ experience in this study differ from treatment outside the study?
- In what ways will this research be novel?
These questions can help you choose the key information for your specific study, but involving people who are like the ones you expect to enroll in the study when creating and assessing the consent form is the best way to further refine the consent. You can do this by:
(1) discussing with people from your intended participant population what might be important to them before you even create the form
(2) usability testing a draft version of the form with these individuals and then making changes in later drafts that are consistent with what you learn. Click here to learn more about usability testing.
Don’t forget: the consent form should “assist a prospective subject or legally authorized representative in understanding the reasons why one might or might not want to participate in the research.” ([46.116(a)(5)(i)])
There is no definite answer for this, so keep in mind the goal – to facilitate understanding. The following tips will help you do so:
- Rank what is most important to participants – This is what will drive most of your decisions. You can categorize things by thinking about what participants must do, risk, or undergo to be in the study. While many are hesitant to try and predict what potential research participants might think is important, trying to put yourself into that mindset, and talking with others outside the medical profession will help you make better ranking decisions.
- Put more important information before less important information (in the document, on pages, and in sections) – people are less likely to overlook something if it is at the beginning.
- Put related content close together – This seems self-explanatory, but you will often see related information scattered throughout longer documents. When the material is not close together, there is a real risk that the participant will not be able to process all of the information in a way that helps make complex decisions.
For example, if you are conducting a study where there is a real risk that the patient may suffer complications, explaining who will pay for the medical treatment if complications arise will be extremely important to most patients. It would, arguably, be more important than if significant results will be provided or if the researcher stands to gain financially.
Step Four – Plan Your Design Strategy
Once you have an outline to help organize the content you will include in your consent form, Step Four is to plan your initial design strategy. Sometimes it is helpful to do this even earlier in the process, especially if you have to make significant choices up front, like whether this consent will be electronic or in print. Determining if this consent will be electronic or in print is the one of the most important factors in determining your design strategy. Whether you use color, the size and page limitations of your consent, and whether you use graphics, media, or other non-text features are all factors that are directly impacted by this decision.
Whatever the medium, there are a number of things that help foster a clear, user-focused design – so you should plan for these before you begin writing a draft:
- Use at least 12-point font (probably larger)
- Use plenty of white space
- Include table, charts, and graphics
- Consider a color scheme with subtle shifts of color to make different sections stand out
- Save enough space for Signature Boxes and Other Logistical Requirements
- Find models of good consent forms to use as a starting point You can find one example here
- Plan time to engage members of your target audience for user-testing and feedback
Note: When using color, be sure to account for participants who are colorblind. Watch out for greens and reds, or any other contrast that makes the content harder to see or read.
Drafting the Content in Plain Language
After diligently planning and preparing to draft a consent form, writing a draft is much easier. Keep your audience in mind as you start writing the content using plain language principles. Let the participants’ need to understand drive your writing decisions. Make sure to check with your IRB/EC to find out if there’s an institutional template you should be using.
If you are not bound by a template, you can find existing templates or consent forms to get ideas on what to say and how to phrase things. But be careful. Most past consent forms are not written health literacy in mind, so alter it to better promote participant understanding using the tips in this guide.
If you must use a specific template, like one from your institution or a sponsor, there are a few reasons why it is still helpful to do your preliminary planning and preparation before turning to the template.
- Think about your audience and purpose for creating the consent form so you can better view the existing template from your prospective participant’s point of view. Many templates were not created with a participant’s health literacy needs in mind. Rather, they were created with an eye towards regulatory compliance. Therefore, as you read through the template, ask yourself whether the wording and the organization would foster participant understanding.
- Use your pre-planned outline to help you assess whether this template covers what you need to cover. Templates have often been used for years, so your planning may alert you that you either have an older version of the template or that the current version is out of date. Plus, while organizations have recently updated their templates to comply with new clinical trial regulations, make sure you are using the most up-to-date version before you start – this will save you time and speed up IRB/EC approval.
- Use your initial design strategy to better determine how to fit your study-specific requirements into the existing template. In any case, if you must use a template, remember your primary purpose under the Revised Common Rule is to facilitate the participant’s decision of whether to take part in the study. Let this be your guide as you decide write and organize the content in the template.
A note about translation: If you plan to consent non-English speakers into your study, translate and back-translate the content to ensure meaning is retained, and then perform usability tested with native speakers of the language.
After you finish planning and writing the first draft of the consent form, there are still important parts left to make it understandable. You must:
- edit the consent form
- test it with your audience
- revise the form based on the testing
Editing before you test helps to ensure that you covered all the needed elements of the consent form Common Rule and followed through on your initial plan for organizing and ordering the information. It also helps to make sure that your headings are consistent and that your consent language follows health literacy best practices. In short, you don’t want to test an unedited draft, you want to reread and polish it to make sure people aren’t distracted when testing it.
One tip for editing effectively is to edit multiple times looking for specific things. If you look for everything in one edit, you will miss a lot. For example, if you do one edit just for the headings, you are more likely to catch every error related to the headings than if you looked for content coverage, headings, word choice, punctuation, etc.
Usability testing with people who are similar to your intended study population will help you spot gaps in content, learn what people think is important, and recognize areas where people may struggle with the writing. Learn more about usability testing .
Revising content is more inclusive than editing content. Revising involves reorganizing content, major rewrites or design changes, and other larger-scale issues. While you may need to revise an early draft of the consent to get it into a form that you can test, it is important to plan on revising content after you test it with your audience. This is when you have actual user input, so your revisions will be much more focused and useful than those revisions you do before testing.
(1) Keep your words, sentences, and paragraphs short (15-25 words per sentence; 3-5 sentences per paragraph, on average). Use bullets as much as possible. Along those lines, use words with fewer syllabus instead of longer words with more syllabus.
(2) Use high-frequency words that people have likely heard before in their everyday lives.
(3) Carefully consider how you use numbers. This is such an important area that we’ve created a separate page to help further explain it.
(4) Explain complex terms. If you must use a complicated term, explain it. People will appreciate it even if it makes the document longer. You can do this by:
- Using visuals to help explain the term or concept.
- Providing a definition or an example using a few short, simple sentences
- Using simpler synonyms, if they exist.
- Tip: There are a few plain language dictionaries that can help give you ideas of clearer terms to use. You can find some of our favorites here
(5) Use the active voice which is more clear and easy to understand. You can find some examples here.
(6) Avoid nominalizations. Nominalizations arise when you create a noun based off of an existing verb or adjective. When this happens, it generally makes for longer words and longer sentence that often lack a clear action. Change the sentence to use the verb or adjective form of the word instead.
| Nominalization | Verb Form |
| decision | decide |
| discussion | discuss |
| removal of | remove |
| disagreement | disagree |
| collection | collect |
(7) Use informative headings. Informative headings tell the participant what a section will be about and how it relates to them. A great way to make a heading more informative is to phrase it as a question or direction rather than a statement.
| Statement | Question | |
|---|---|---|
| Risks | vs. | What are the risks of being in the study? |
| Injury | vs. | What happens if I get injured? |
| Study Procedures | vs. | What will happen if I join the study? |
(8) Break information into shorter chunks of information – this helps make the information more digestible.
(9) Include enough white space so that text is not overwhelming – it has to look inviting for people to want to start reading it.
(10) Use clear visuals instead of just words – we know people learn in different ways, so use images, graphics, and video to help explain topics that are difficult to explain using words.