By: Carolynn Thomas Jones
To address the need for a well-prepared, competent workforce, the National Center for Clinical and Translational Science (NCATS) has funded a grant entitled “Development, Implementation, and Assessment of Novel Training in Domain-based Competencies” (DIAMOND). This grant has four multi-PIs at University of Michigan, The Ohio State University, Tufts University and University of Rochester, and is a continuation of earlier work funded by NCATS called “Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ)” (1). These two projects have both embraced the JTF Clinical Trial Competencies, including applications of the competency domains beyond clinical trials to include social and behavioral clinical research. Individuals from the Joint Task Force were highly involved in workgroups for ECRPTQ (Stephen Sonstein, Carolynn Jones and Jonathan Seltzer, and in DIAMOND (Carolynn Jones) serves as mPI and Stephen Sonstein serves as an Advisory Board Member.
JTF Core Competency Framework Actively Promoted Through Association Of Clinical Research Professionals In Mexico (APEIC)
October 15, 2018
The Association of Clinical Research Professionals in Mexico (APEIC) is actively promoting the JTF Core Competency Framework among local clinical research professionals and the different local associations. Academic sessions based upon the JTF domains are being presented at monthly meetings and a professional certification program based upon the core competencies is being developed targeting APEIC associates. The JTF Competency Domain “wheel” has been translated into Spanish and is now used in all APEIC public messages (more…)
JTF Presentations At 19th Annual Conference Of Pharmaceutical Medicine, Tokyo, Japan
October 15, 2018
On September 28, 2018, JTF members, Honorio Silva and Stephen Sonstein took part in a symposium, “Education in Pharmaceutical Medicine and Clinical Research” as part of the 19th International Conference of Pharmaceutical Medicine in Tokyo, Japan. The symposium presentations discussed the progress made in defining core competencies for medicines development and clinical research, described the educational needs of drug development scientists and clinical research professionals, and described initiatives for accreditation of academic programs and certification of clinical research professionals. The overheads from the three presentations are presented below:
Joint Task Force for Clinical Trial Competency
International Perception of Competence – Needs for Clinical Research Education
First Academic Program In Clinical Research Accredited
October 01, 2018
ACRP Supports The Use Of The Joint Taskforce For Clinical Trial Competency Framework As The Standard For Clinical Research Workforce Development
July 03, 2018
There is a critical shortage of qualified professionals in the clinical research enterprise and a need to have standard methodology and competency levels for clinical researchers. Jim Kremidas, executive director of ACRP, addresses this critical issue in his article “A Turning Point: Examining the clinical research workforce in 2018” and calls for the Harmonized Core Competency Framework of the Joint Task Force for Clinical Trial Competency, as the standard to be utilized for development of the clinical trial workforce. Read More
Joint Task Force For Clinical Trial Competency Finalist For ACRP Innovation In Workforce Development Award
May 30, 2018
The Joint Task Force for Clinical Trial Competency (JTF) was one of three finalists for the Innovation in Workforce Development Award of the Association of Clinical Research Professionals (ACRP). The JTF was formed in 2013 by bringing together many voices and perspectives to develop across-the-board standards that are applicable for clinical trial professionals throughout the clinical trial process. The JTF has been a driving force to change the dynamic of hiring standards from being based on years of experience to being based on competencies and for education and training to be competency-based. ACRP Blog Post
Ensuring Representativeness In Competencies For Research Coordinators
May 30, 2018
Clinical Researcher—May 2018 (Volume 32, Issue 5)
Providing educational programs to develop clinical research coordinators’ (CRCs’) competency skills is essential to workforce development, yet little is known about how programs address CRCs’ needs. Frameworks have been developed to guide training programs that focus on the implementation and application of competency-based skills in research coordination and management. One of these, the Joint Task Force for Clinical Trial Competency (JTF) framework, is intended to be broad and applicable to individuals conducting, supporting, and managing research in varied professional capacities. (more…)
Duke University School Of Medicine Uses JTF Competencies To Reduce Number Of Job Descriptions From 80 To 12
April 02, 2018
The field of clinical research has changed considerably in the past 20 years, and staff supporting the research are asked to take on additional responsibilities, learn new processes, and be continuously educated on modernized policies and procedures. To address the increased responsibilities and complexities of work, Duke University School of Medicine leadership agreed that an overhaul of job descriptions for clinical research professionals was needed. A working group was created, assembling administrative leaders, human resources personnel, and clinical research subject matter experts. The group used the competencies developed by the Joint Task Force for Clinical Trial Competency as the foundation for this work. Ultimately, they simplified the number of job classifications at Duke from approximately 80 to 12 and mapped over 700 employees into the new jobs. They recently published a paper which describes the effort and the lessons learned. To review the paper click this link.
The UK Integrated Workforce Framework: NIHR
October 26, 2017
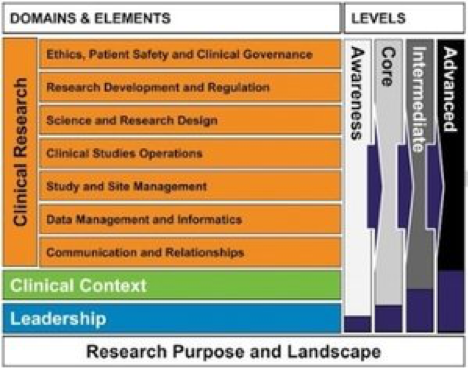
The UK National Institute for Health Research(NIHR) Clinical Research Network (CRN) has used the JTF Framework in a clinical research workforce development initiative called the Integrated Workforce Framework (IWF). This is part of a national project that included input from a selection of stakeholders and endorsed by a project board from the UK Department of Health. This was built upon existing resources that were tried and tested, including the Royal College of Nursing Framework for Clinical Research Nurses (2011) and the Joint Task Force Clinical Research Core Competency Framework (Sonstein et al, 2014). Read more
Core Competency Framework Version 2.0
September 01, 2017

