12 articles about building the clinical research workforce
March 22, 2024
The journals Frontiers in Medicine and Frontiers in Pharmacology have recently published 12 articles on the research topic: Building the Clinical Research Workforce: Challenges, Capacities and Competencies. The articles cover the following topics:
– Clinical research roles and professionalism
– Clinical research academic education, training and competency development, assessment
– Clinical research professional employment, retention, progression
– Diversity of the clinical research workforce
– Applications and contributions of the JTF Framework
– Clinical research workforce development in the setting of the evolving clinical research paradigm
– Clinical research team science, defining research teams, workforce spectrum
The following articles and an overview of the research topic can be accessed on this website.
DIAMOND Portal
October 23, 2023
The National Center for Advancing Translational Sciences (NCATS) highlighted the JTF on the DIAMOND Portal, a portal that provides access to training materials for clinical research professionals and others on the research team. DIAMOND stands for Development, Implementation, and Assessment of Novel Training in Domain-based Competencies.
The DIAMOND Portal, brings together trainings in the eight competency domains of the JTF Framework, ranging from the specifics of running trials to more general topics, such as leadership and professionalism. The portal also includes assessments that study teams can use to assess the training of staff members. In addition, research professionals can boost their careers by maintaining an electronic portfolio of their training materials through DIAMOND. While the initiative began as a way to share materials developed at the CTSA Program hubs, now additional resources can be uploaded to the portal.
ACRP presented on workforce development and interrelation with JTF
June 02, 2023
At the April 2023 annual meeting of the Association of Clinical Research Professionals (ACRP), Susan Landis and Erika Stevens presented on workforce development in clinical research and its interrelationship with the JTF Core Competency Framework. A blog on the topic can be found here.
JTF Framework used to develop certificate program
September 09, 2022
In order to fill the gap in junior investigator training, Tufts University Clinical and Translational Science Institute (CTSI) has utilized the JTF Core Competency Framework to develop an eight-module, certificate program to teach aspiring clinician-investigators about good clinical practice, clinical research processes, and federal and local regulatory requirements.
Results of global survey of clinical research professionals
May 26, 2022
Recently, the JTF published in Therapeutic Innovation & Regulatory Science the results of a global survey of clinical research professionals who were asked to self-assess their level of competency for each of the 8 domains of the JTF Framework. The results were very similar to those of a similar 2016 published JTF survey showing that irrespective of role, years of experience or educational level, lower levels of competency were expressed in the domains of Scientific Concepts and Research Design and Investigational Product Development and Regulation. The recent survey showed an additional lower level of competency for the Data Management and Informatics domain. Respondents with professional certification in clinical research self-assessed their competency at higher levels in all domains than those without professional certification.
Incorporated Competencies Related to Project Management into JTF Framework
January 21, 2022
Recently published in Therapeutic Innovation & Regulatory Science, the Joint Task Force for Clinical Trial Competency (JTF) defined and documented core competencies related to clinical project management and project management skills. Two new specific competencies with related examples were incorporated in Version 3.1 of the JTF Framework and the wording of several existing competencies was modified.
January 14, 2022
Communication and Teamwork in JTF Framework
This article focuses on Domain 8 of the JTF Framework – Communication and Teamwork. The authors have emphasized the importance of communication skills between Clinical Research Professionals and the perception of competence.
January 14, 2022
Duke University integrating the JTF Framework into is clinical research enterprise
Duke University School of Medicine and Nursing has been an innovator in integrating the JTF Framework into its clinical research enterprise. This article discusses the impact of classifying clinical research roles according to the JTF Competency Framework on workforce turnover in an academic medical center. Significant reduction in personnel turnover was noted following the reclassification and reduction in number of defined roles.
International Study of clinical investigator competence by AIDS Malignancy Consortium
October 07, 2021
The AIDS Malignancy Consortium (AMC) conducted a survey of clinical investigators in Latin America and Sub-Saharan Africa to determine self-assessed competency, utilizing the JTF Clinical Research Core Competency Framework, to inform training and professional development needs. The results were similar to prior findings by the JTF (Clinical Researcher, 2016:3;42-49) showing lower levels of competency in the domains of Scientific Concepts and Research Design, Medicines Development and Regulation and Data Management and Informatics. The AMC is using the JTF Framework to develop a training curriculum tailored to the needs of their clinical staff.
Virginia Commonwealth University initiates workforce development and Post-baccalaureate certificate programs based upon JTF Core Competency Framework
January 22, 2021
Virginia Commonwealth University (VCU), C. Kenneth and Dianne Wright Center for Clinical and Translational Research Translational Workforce Development Program relies on the JTF Framework for overall workforce development activities, provides the JTF Framework as an ongoing education and training resource to the workforce, aligns Clinical Research Skills Series workshop learning objectives to the core competencies, and designed the Post-baccalaureate Certificate in Clinical Research core and elective courses requirements based on the framework competencies. cctr.vcu.edu
A Perspective on the Current State of Clinical Research Education and Training
December 02, 2020
In this peer-reviewed article, authors Joseph M. Bocchino, EdD, Joan Butler, EdD, MS, and Beth Harper, MBA, BS (OT), review the literature addressing the current state of education within the clinical research profession to ascertain directions in which the profession and its supporting educators may be moving in order to further develop clinical research workforce capacity. The importance of competency-based education and the JTF Core-Competency Framework are highlighted.
Professional Development for Clinical Research Professionals: Implementation of a Competency-Based Assessment Model
October 21, 2020
Duke University’s Office of Clinical Research has been on the forefront of utilizing the JTF Core competency Framework to define its role descriptions as well as to clearly specify how a clinical research professional can move upwards on their career ladders. Their recent publication through the Society of Research Administrators International (SRAI), Volume LI, Number 2 explains these processes in detail. The authors conclude that the utilization of the JTF Framework in this manner has benefited the institution by increasing CRP retention rates and allowed Duke to better understand current competency levels of its workforce. Duke University’s Office of Clinical Research and their utilization of the JTF Core Competency Framework has been a model for the academic community and their efforts have been utilized by several of the Clinical and Translational Science Award institutions.
Assessing Clinical Investigators Perceptions of Relevance and Competency of Clinical Trials Skills: An international AIDS Malignancy Consortium (AMC) Study
August 12, 2020
A paper appearing in the Journal of Clinical and Translational Research reported on a study by the International AIDS Malignancy Consortium which assessed the competency level and role relevance of the JTF Core Competencies in Clinical Investigators from Sub-Saharan Africa and Latin America. The results indicated that the highest relevance to role was in the Scientific Concepts and Research Design and the Ethical and Participant Safety domains and the lowest in the Data Management and Informatics domain. A high level of self-assessed competency was seen in the Ethical and Participant Safety domain, but lower levels of self-assessed competency were seen in the Data Management and Informatics and the Scientific Concepts and Research Design domains. An additional domain, Community Engagement, was added to the framework by the investigators due to the perception of increased relevance in the assessed geographic regions, though interestingly, this domain showed a high level of competence, but a low level of role relevance. The AMC is working to incorporate professional development offerings to investigators based upon the results of this assessment.
Publication of Leveled JTF Core Competency Framework in Japan
July 17, 2020
Our Japanese colleagues have published the Joint Task Force Core Competency Framework in the Japanese Journal of Clinical Pharmacology and Therapeutics (2020;51(3):131-150). This publication in the Japanese language helps extend the integration of the Framework to clinical research professionals in Japan.
New ACRP Program to Assess Competency of Entry-Level Clinical Researchers
May 06, 2020
The Association of Clinical Research Professionals (ACRP) announces a pilot project designed to assess the competency of entry-level clinical research professionals, ACRP’s hypothesis is that new graduates from academic programs in clinical research will pass the ACRP-CP exam at equal or higher rates than individuals with a minimum of 2 years’ experience in clinical research. If proven true, then providing graduating students access to an ACRP-CP certification exam will enable the further demonstration of their knowledge and competency thereby enhancing a hiring organization’s confidence in recruiting, training, and retaining these new graduates as valuable members of their research teams. The Consortium of Academic Programs in Clinical Research has been working for some time with ACRP to develop this program. Three institutions are participating in the program, Campbell University, University of North Carolina Wilmington and Durham Technical Community College.
Academic Program Accreditation: An Important Criterion for Quality in the Preparation of Tomorrow’s Clinical Research Workforce
February 07, 2020
Indices of clinical research coordinators’ competence
August 05, 2019
A recent publication reports that investigators from the Clinical and Translational Science Award based DIAMOND project have developed and tested indices which may be used to evaluate the self-assessed competency of clinical research coordinators. The two indices, CICRP-I (basic level) and CICRP-II (advanced level) were developed using data selected from the JTF Global Self-Assessment of Clinical Research Competency. The CICRP-II assessment was administered to 95 clinical research coordinators working in one of 4 CTSA funded academic health centers in an effort to accumulate data which could be used to confirm the validity of the instrument and compare the results to similar assessments which were published by the JTF that self-assessed the competency of a variety of members of the global clinical research enterprise.
Career Orientation and perceived professional competence among clinical research coordinators
August 02, 2019
A recent publication by faculty from the University of Georgia and Emory University described a study of how the JTF Core Competency Domains are perceived by clinical research coordinators as relates to their career orientation and career satisfaction. Different aspects of the role of clinical research coordinator define certain career orientation types. Four different types were identified utilizing a web-based survey. The conclusion of the study was that “understanding the career orientation of CRC’s can be helpful to institutional administrators and clinical investigators as they support professional development and training of CRC’s.
Core Competencies Applied: Duke University School of Medicine
August 02, 2019
 The Harmonized Core Competency Framework was utilized in a major workforce development effort at Duke University. Rebecca Brouwer (Associate Director for Research Operations at Duke Office of Clinical Research) and Denise Snyder (Associate Dean for Clinical Research at Duke School of Medicine) spearheaded this effort to reclassify all research professional positions within Duke University. (more…)
The Harmonized Core Competency Framework was utilized in a major workforce development effort at Duke University. Rebecca Brouwer (Associate Director for Research Operations at Duke Office of Clinical Research) and Denise Snyder (Associate Dean for Clinical Research at Duke School of Medicine) spearheaded this effort to reclassify all research professional positions within Duke University. (more…)
Opportunity to Define Competency Standards for the Clinical Project Manager
February 22, 2019
ACRP Presentation To CTSN Clinical Research Professional Taskforce Highlights JTF Core Competency Framework
January 30, 2019
ACRP’s Workforce Innovation Officer, Beth Harper, recently addressed the Clinical and Translational Science Network Professional Taskforce and discussed Competency Based Career Progression. The presentation discussed the evolution of competency based hiring, retention and career progression and stressed the need for standards and the value of professional certification for the clinical research workforce and highlighted the contributions of the Joint Taskforce for Clinical Trial Competency. Her presentation slides are attached.
Forte Research Systems, Inc. Hosts Webinar, “Competency-Based Approaches To Staff Hiring, Management And Advancement”
November 14, 2018
On November 6, 2018, Beth Harper, Workforce Innovation Officer for the Association of Clinical Research Professionals, hosted a webinar which was sponsored by Forte Research Systems, Inc of Madison, Wisconsin. The webinar overviewed the development of the JTF Core Competency Framework and the impact that it was having on clinical research staff hiring, training and upward mobility. — As of 2019, Forte has merged and is now part of Advarra.
New Opportunity To Define Competency Standards For The Clinical Project Manager
November 05, 2018
The DIAMOND Portal
October 15, 2018
By: Carolynn Thomas Jones
To address the need for a well-prepared, competent workforce, the National Center for Clinical and Translational Science (NCATS) has funded a grant entitled “Development, Implementation, and Assessment of Novel Training in Domain-based Competencies” (DIAMOND). This grant has four multi-PIs at University of Michigan, The Ohio State University, Tufts University and University of Rochester, and is a continuation of earlier work funded by NCATS called “Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ)” (1). These two projects have both embraced the JTF Clinical Trial Competencies, including applications of the competency domains beyond clinical trials to include social and behavioral clinical research. Individuals from the Joint Task Force were highly involved in workgroups for ECRPTQ (Stephen Sonstein, Carolynn Jones and Jonathan Seltzer, and in DIAMOND (Carolynn Jones) serves as mPI and Stephen Sonstein serves as an Advisory Board Member.
JTF Core Competency Framework Actively Promoted Through Association Of Clinical Research Professionals In Mexico (APEIC)
October 15, 2018
The Association of Clinical Research Professionals in Mexico (APEIC) is actively promoting the JTF Core Competency Framework among local clinical research professionals and the different local associations. Academic sessions based upon the JTF domains are being presented at monthly meetings and a professional certification program based upon the core competencies is being developed targeting APEIC associates. The JTF Competency Domain “wheel” has been translated into Spanish and is now used in all APEIC public messages (more…)
JTF Presentations At 19th Annual Conference Of Pharmaceutical Medicine, Tokyo, Japan
October 15, 2018
On September 28, 2018, JTF members, Honorio Silva and Stephen Sonstein took part in a symposium, “Education in Pharmaceutical Medicine and Clinical Research” as part of the 19th International Conference of Pharmaceutical Medicine in Tokyo, Japan. The symposium presentations discussed the progress made in defining core competencies for medicines development and clinical research, described the educational needs of drug development scientists and clinical research professionals, and described initiatives for accreditation of academic programs and certification of clinical research professionals. The overheads from the three presentations are presented below:
Joint Task Force for Clinical Trial Competency
International Perception of Competence – Needs for Clinical Research Education
First Academic Program In Clinical Research Accredited
October 01, 2018
ACRP Supports The Use Of The Joint Taskforce For Clinical Trial Competency Framework As The Standard For Clinical Research Workforce Development
July 03, 2018
There is a critical shortage of qualified professionals in the clinical research enterprise and a need to have standard methodology and competency levels for clinical researchers. Jim Kremidas, executive director of ACRP, addresses this critical issue in his article “A Turning Point: Examining the clinical research workforce in 2018” and calls for the Harmonized Core Competency Framework of the Joint Task Force for Clinical Trial Competency, as the standard to be utilized for development of the clinical trial workforce. Read More
Joint Task Force For Clinical Trial Competency Finalist For ACRP Innovation In Workforce Development Award
May 30, 2018
The Joint Task Force for Clinical Trial Competency (JTF) was one of three finalists for the Innovation in Workforce Development Award of the Association of Clinical Research Professionals (ACRP). The JTF was formed in 2013 by bringing together many voices and perspectives to develop across-the-board standards that are applicable for clinical trial professionals throughout the clinical trial process. The JTF has been a driving force to change the dynamic of hiring standards from being based on years of experience to being based on competencies and for education and training to be competency-based. ACRP Blog Post
Ensuring Representativeness In Competencies For Research Coordinators
May 30, 2018
Clinical Researcher—May 2018 (Volume 32, Issue 5)
Providing educational programs to develop clinical research coordinators’ (CRCs’) competency skills is essential to workforce development, yet little is known about how programs address CRCs’ needs. Frameworks have been developed to guide training programs that focus on the implementation and application of competency-based skills in research coordination and management. One of these, the Joint Task Force for Clinical Trial Competency (JTF) framework, is intended to be broad and applicable to individuals conducting, supporting, and managing research in varied professional capacities. (more…)
Duke University School Of Medicine Uses JTF Competencies To Reduce Number Of Job Descriptions From 80 To 12
April 02, 2018
The field of clinical research has changed considerably in the past 20 years, and staff supporting the research are asked to take on additional responsibilities, learn new processes, and be continuously educated on modernized policies and procedures. To address the increased responsibilities and complexities of work, Duke University School of Medicine leadership agreed that an overhaul of job descriptions for clinical research professionals was needed. A working group was created, assembling administrative leaders, human resources personnel, and clinical research subject matter experts. The group used the competencies developed by the Joint Task Force for Clinical Trial Competency as the foundation for this work. Ultimately, they simplified the number of job classifications at Duke from approximately 80 to 12 and mapped over 700 employees into the new jobs. They recently published a paper which describes the effort and the lessons learned. To review the paper click this link.
The UK Integrated Workforce Framework: NIHR
October 26, 2017
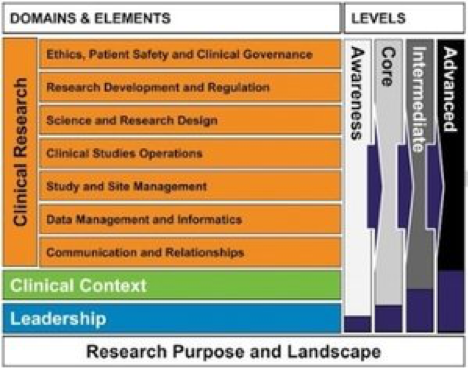
The UK National Institute for Health Research(NIHR) Clinical Research Network (CRN) has used the JTF Framework in a clinical research workforce development initiative called the Integrated Workforce Framework (IWF). This is part of a national project that included input from a selection of stakeholders and endorsed by a project board from the UK Department of Health. This was built upon existing resources that were tried and tested, including the Royal College of Nursing Framework for Clinical Research Nurses (2011) and the Joint Task Force Clinical Research Core Competency Framework (Sonstein et al, 2014). Read more
Core Competency Framework Version 2.0
September 01, 2017
Core Competencies Applied: PRAXIS Australia
August 31, 2017
PRAXIS Australia has used the Harmonized Core Competency Framework to create flexible learning opportunities for the Australian research sector.
PRAXIS Australia Ltd (PRAXIS) is an Australian not for profit/charity organisation, founded by two of Australia’s leading academic institutions, University of Sydney and Monash University (via another NGO, Global Reconciliation Ltd), and the largest provider of independent ethics review in Australia, Bellberry Limited. Their mission is to enhance the understanding and practice of ethical research for the benefit of the broader community, via the provision of various training options, underpinned by two flagship training models – HREC Essentials and Research Essentials.
Core Competencies Applied: Committee On Accreditation Of Academic Programs In Clinical Research
April 26, 2017
The first academic programs in Clinical Research were developed in the late 1990s. Those early programs were successful in providing a pipeline of new, highly qualified graduates to the clinical research enterprise. With the increasing number and complexity of clinical trials and the severe shortage of qualified new clinical research professionals, these graduates continue to be highly sought after today.
Clinical Research has thus become a recognized academic discipline, and there are more than 50 academic programs in the US and more than 50 in other parts of the world.
Core Competencies Applied: Duke University School of Medicine
March 22, 2017
 The Harmonized Core Competency Framework was utilized in a major workforce development effort at Duke University. Rebecca Brouwer (Associate Director for Research Operations at Duke Office of Clinical Research) and Denise Snyder (Associate Dean for Clinical Research at Duke School of Medicine) spearheaded this effort to reclassify all research professional positions within Duke University. (more…)
The Harmonized Core Competency Framework was utilized in a major workforce development effort at Duke University. Rebecca Brouwer (Associate Director for Research Operations at Duke Office of Clinical Research) and Denise Snyder (Associate Dean for Clinical Research at Duke School of Medicine) spearheaded this effort to reclassify all research professional positions within Duke University. (more…)

