opt-out opt-out

To decline optional study activities or optional data uses that are not required for the study.
Example of opt-out in a sentence
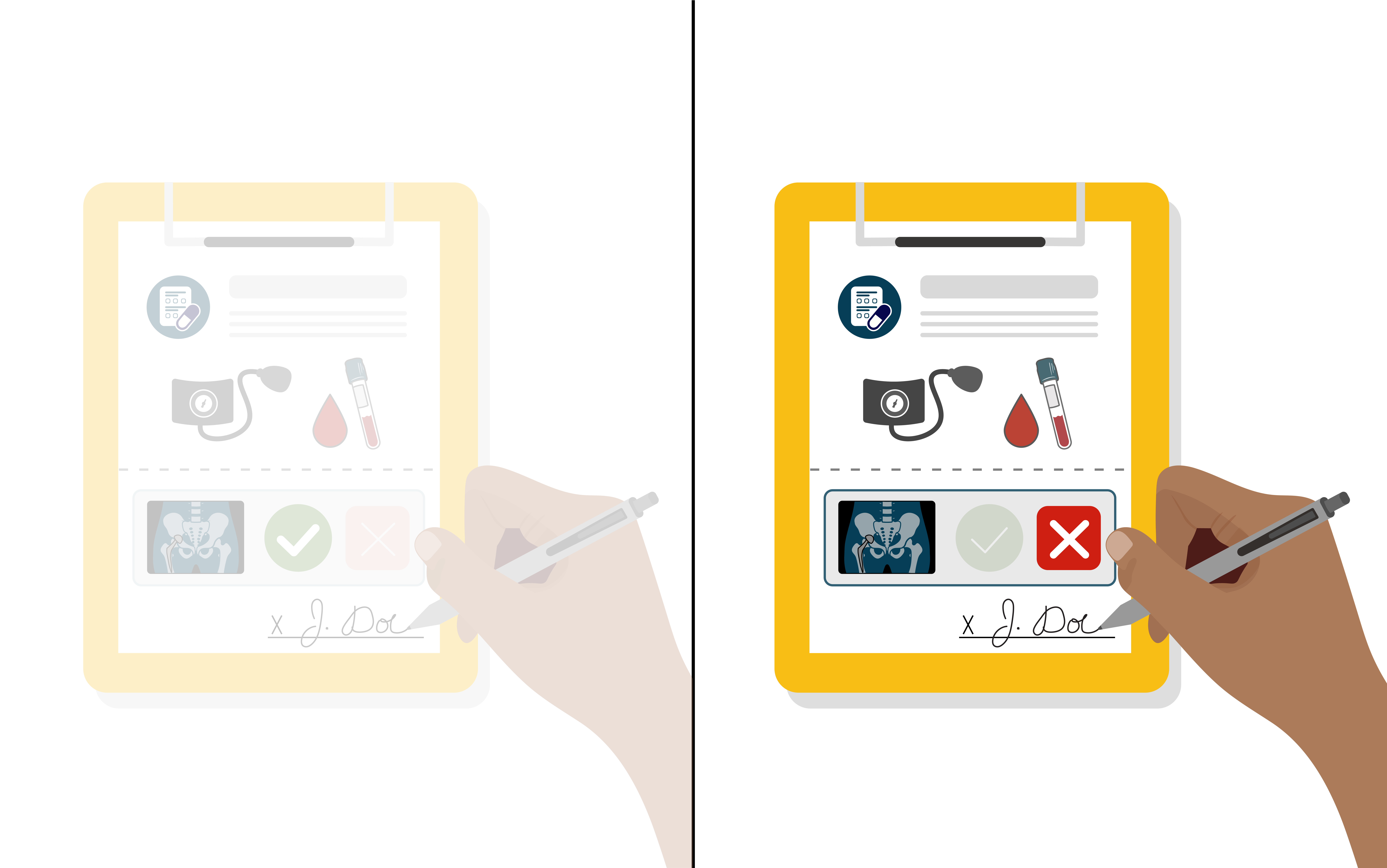
Research consent forms sometimes include check boxes that allow a participant to opt-out of a part of the study.
More Info
When certain study procedures or activities are optional, and the participant chooses not to do them, this is called opt-out.
In some cases, a research study may include specific procedures unless a participant decides to opt-out. For example, a study may include the option to collect spinal fluid, but not require it for someone to be in the study. Or a study may plan to videotape a research interview, but the participant can opt-out of being recorded.
Opt-out is also used to describe when participants choose not to have their data used beyond the original purpose of a research study.
Other info to think about when joining a study
You might see the term “opt-out” used to describe the choice you have to not do optional parts in a study or have your data used beyond the original goals of the study.
If you have any questions about what is required to be in a study, and what activities are optional, please ask the study team.
You always have the right to withdraw from a study if you no longer want to be a participant. You should discuss this choice with the study team so that you can leave the study safely