decentralized clinical trial decentralized clinical trial

A type of research study where some or all of the participant's study-related activities happen at places other than a single study site.
Example of decentralized clinical trial in a sentence
In a decentralized clinical trial, there could be remote data collection, like measuring heart rate through a device provided to the participant.
More Info
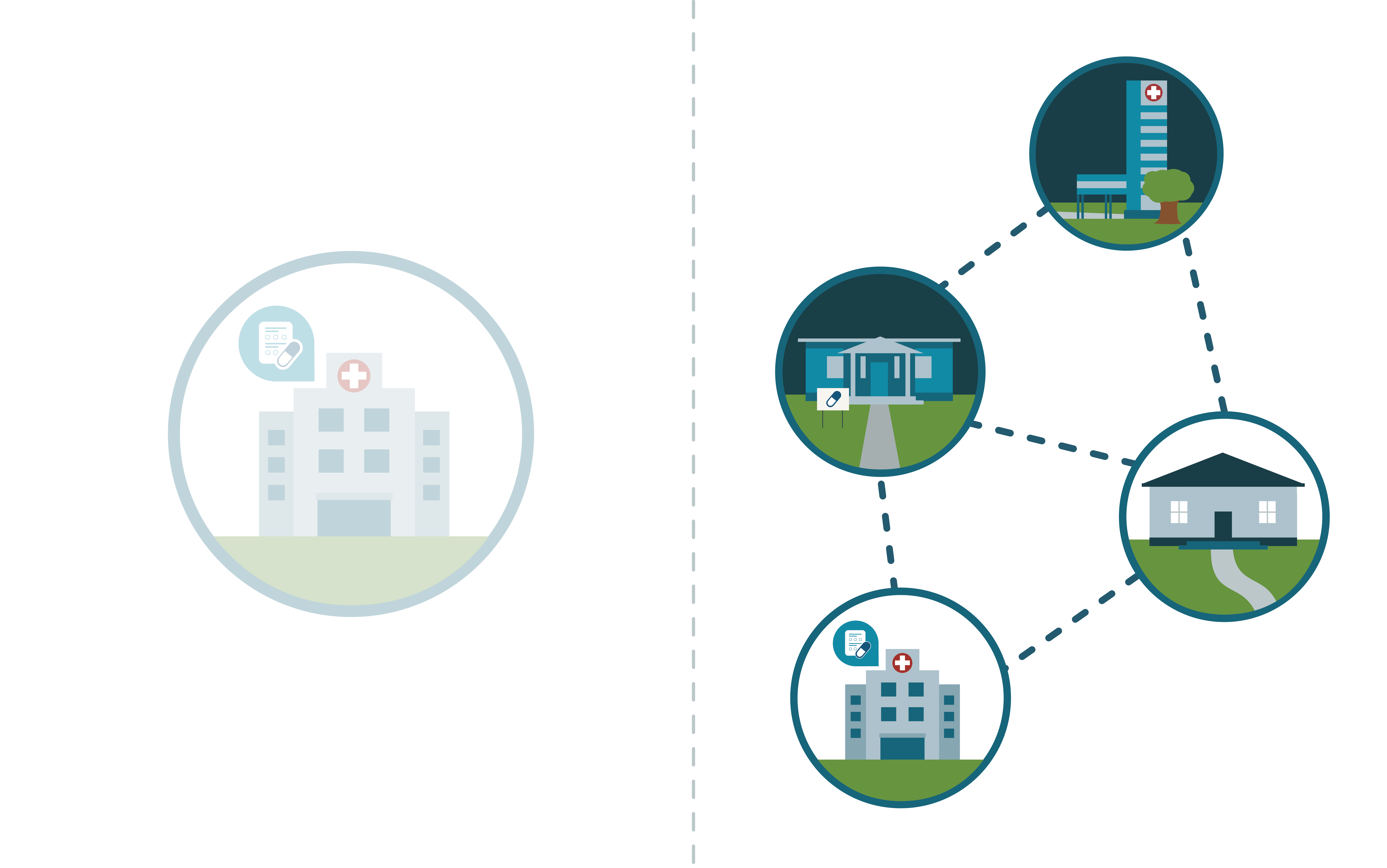
In a decentralized clinical trial (DCT), some or all of the study activities take place away from a central study site.
For example, participants may go through the consent process virtually or have remote visits with their study team using a phone or computer. There could also be home visits by members of the study team.
In a DCT, the participant might also be able to go to a local lab or pharmacy to get a blood draw, instead of going to a single study site for every procedure.
The consent form usually describes which parts of the study are allowed to be done in different locations.
Other info to think about when joining a study
If you are thinking about joining a decentralized clinical trial, the consent form will explain what will happen in the study and where study procedures could take place. You should feel free to ask the study team any questions about what is expected and what they will provide in order to be in the study.
You can ask questions about how you will interact with the study team during the study and how you can get help with being in the study if needed. For example, if the decentralized clinical trial uses a device or new technology to collect data, you should ask who to contact if they aren’t working for you.
You can also ask if there are any options to meet the study staff in person if necessary.