Global Regulatory Engagement
Regulatory issues in different countries and in different regions of the world impact the conduct, efficiency, and oversight of clinical trials. Our approach has been to work with representatives of the global clinical trial ecosystem to identify major issues in international clinical trial practice. We strive to promote a country-specific and culturally relevant approach.
In today’s global environment, there are numerous challenges to expanding clinical research sites globally, such as differing regulations and processes, variability in staff availability and training, inadequate hospital/clinical sites and laboratories, lack of supply chain infrastructure, concerns related to drug shipping/storage, currency devaluation, and governmental instability. Collaborative and harmonized efforts are critical for building capacity in clinical research in these new geographic regions. There is a need for targeted engagement with regulators, national ethics committees, and influential thought leaders to develop trusted partnerships with a shared mission of improving the ethics, design, conduct, and oversight of multi-regional clinical trials. The MRCT Center is working with diverse stakeholders to advance clinical research in new geographic regions, including Africa, the Middle East, Southeast Asia, and Latin America.
Objectives
- Work closely with in-country leaders to determine tractable solutions to regulatory reform issues and ethics review
- Collaborate with clinical trial stakeholders to understand and harmonize efforts to build capacity in clinical research
- Build individual and systems capacity for regulatory and ethics review, cooperation, and reliance
- Help establish sustainable infrastructure, operations, and expertise to enable training and continuous learning
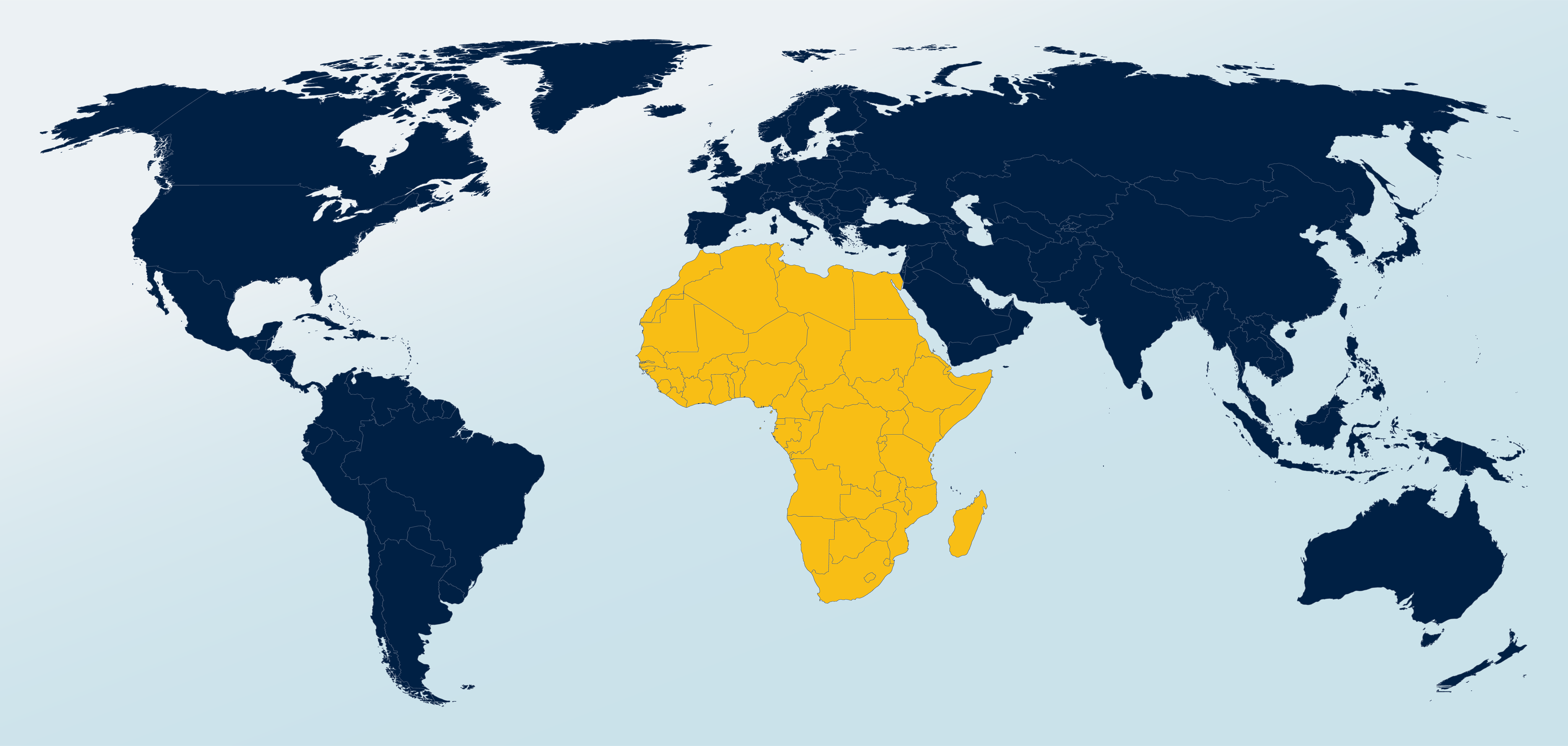
Building Capacity for Ethics Review of Clinical Research Across Africa
Collaborating with international and local organizations to build capacity for research ethics committees across Africa and worldwide. For more information, click here.
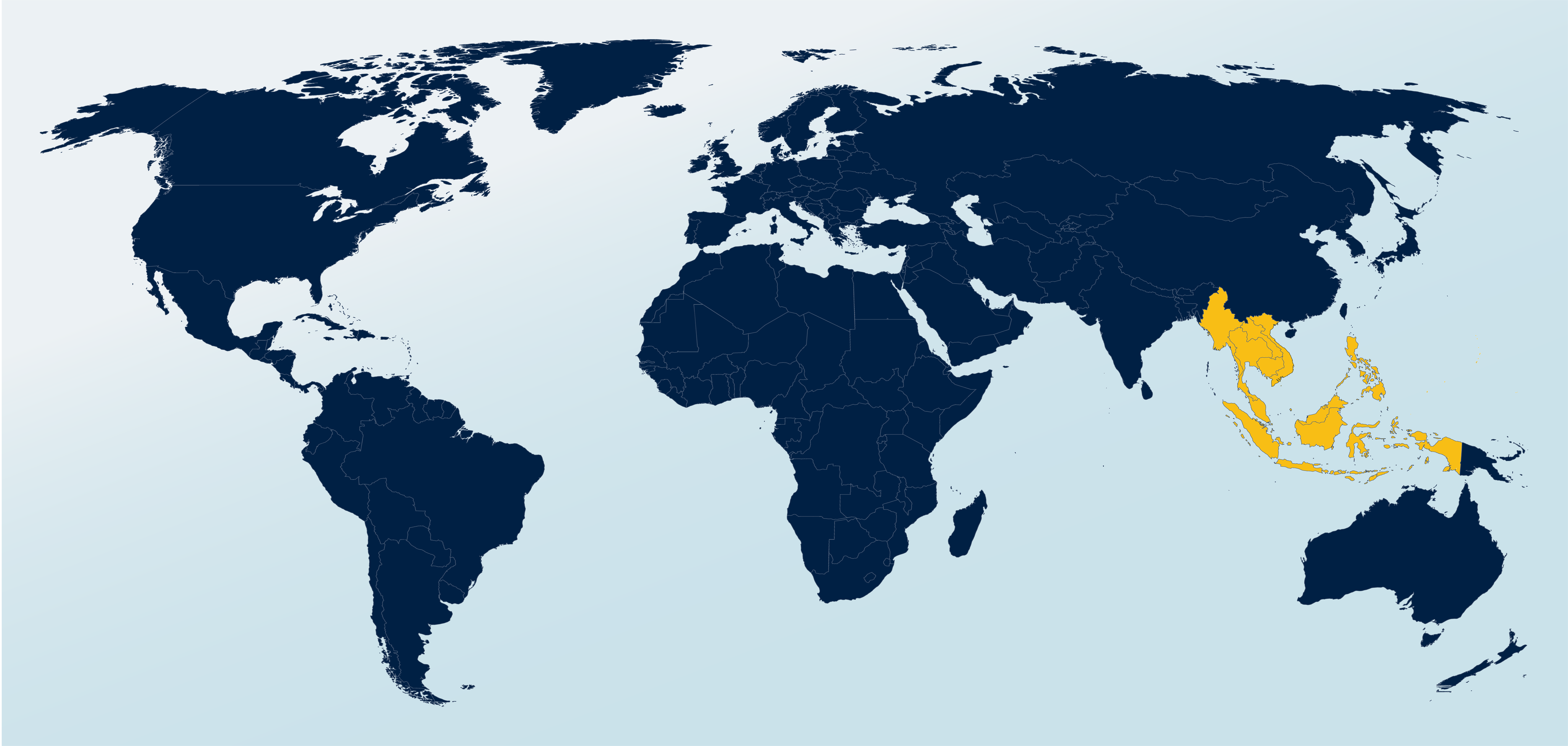
Building Capacity for innovative multi-country clinical trials in South-East Asia
The MRCT Center has worked together with academic institutions in South Korea to build capacity for clinical trials for more than a decade. For half a decade, the MRCT Center has collaborated on an innovative approach to setting up a three-country study in South-East Asia involving integrative therapies with a common endpoint. The MRCT Center developed a self-monitoring toolkit and provides recommendations on study design and expert guidance to support optimal study conduct.
Key Milestones related to the MRCT Center’s work in South-East Asia
- January 2025: Published an article about “Analysis of the choice of control in acupuncture clinical trials involving patients with breast cancer”
- April 2023: Published article about “Conducting a three-country clinical trial during the COVID-19 pandemic: experience and future considerations”
- June 2022: Started providing remote support for monitoring the performance of an international interventional trial on preventing chemotherapy-induced peripheral neuropathy with acupuncture
- June 2022: Published article about “Developing and implementing a self-monitoring toolkit for a coordinated multinational randomized acupuncture trial”
- 2019-2021: Provided remote support for monitoring the performance of a coordinated three-country study on reducing or preventing chemotherapy-induced hot flashes in breast cancer patients
- March 2019: Prepared a self-monitoring toolkit for remote monitoring of a three-country acupuncture trial
Contact mrct@bwh.harvard.edu for more information.
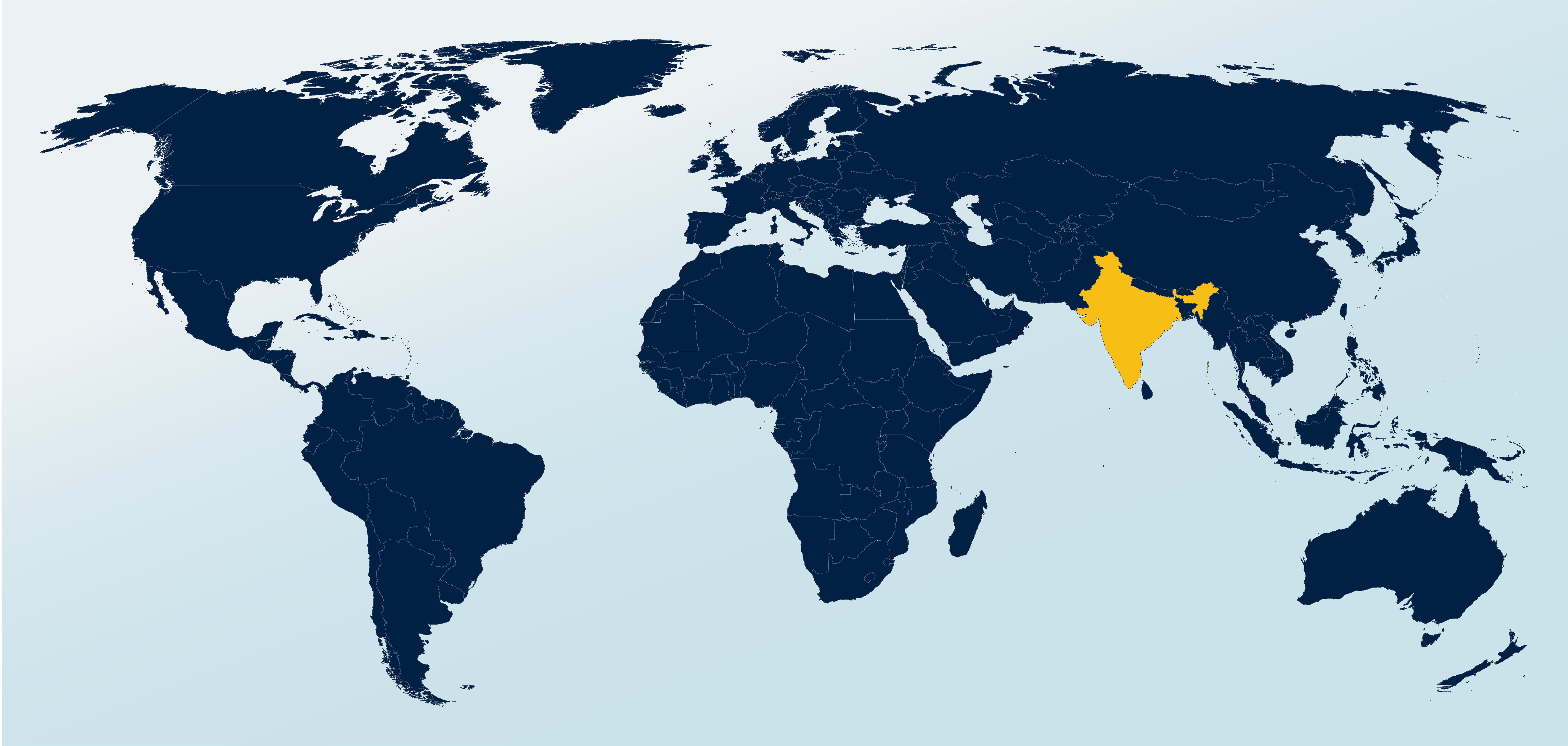
Regulatory Reform in India
From 2013 to 2019, the MRCT Center worked closely with leaders in India to determine tractable solutions to regulatory reform issues, many of which deterred both for-profit and not-for-profit sponsors from conducting trials in the country. The MRCT Center worked with government officials, industry stakeholders, investigators, and others to develop alternative reforms that would not only reinvigorate the industry but also ensure the welfare of trial participants.
Key Milestones related to the MRCT Center’s work in India
- July 2019: Published article with update about the evolving regulatory landscape for clinical trials in India
- May 2019: The MRCT Center, in collaboration with Ropes & Gray LLP, hosted a special presentation and discussion with the Drugs Controller General of India to discuss the final New Drugs and Clinical Trials Rules, 2019, that were released in March, 2019 by India’s Ministry of Health and Family Welfare
- March 2019: New rules released in India
- December 2018: Published article about the evolving regulatory landscape in India
- June 2018: Meetings with regulators, investigators, and industry in Delhi and Mumbai, India
- March 2018: Submitted comments on updated draft rules in India
- May 2017: Submitted comments on draft rules in India
- March 2017: Additional meetings with representatives in India
- March 2016: Meetings with regulators, investigators, representatives of industry, academia, non-profit organizations and patient advocacy groups in Delhi and Mumbai, India
- October 2015: Meetings with regulators and thought leaders in Delhi, India
- November 2014- January 2015: Publications in Financial Express, Business Line, and Bloomberg BNA related to clinical trial regulations in India and proposed amendments to Drugs and Cosmetics Act.
- January 2014: Output of January 20 and 21, 2014, Roundtable included a draft of detailed recommendations embodying the consensus of different stakeholders, including leading academicians, investigators, industry representatives, and government officials. This document also suggested additional issues that the Ministry of Health and Family Welfare, Government of India, may wish to reconsider when implementing clinical research regulatory reform in India.
Contact mrct@bwh.harvard.edu for more information.
Multi-Regional Clinical Trial Guidance in China
From 2015 to 2017, in response to the China Food and Drug Administration (CFDA) issuing a Draft Guidance on Multi-Regional Clinical Trials that set forth requirements for clinical trials in China, the MRCT Center, in collaboration with Peking University, developed scientific principles upon which to ground decision-making for MRCTs.
Key Milestones related to the MRCT Center’s work in China
- March 2017: Patient-Centric Clinical Trials Conference in Shanghai
- September 2016: China FDA Training at Yale University
- May 2016: Global Simultaneous Drug Development Workshop in Beijing
- October 2015: Workshop in Beijing
- June 2015:
◦ Roundtable in Shanghai
◦ Launched Consistency and Region Working Group - April 2015: First roundtable teleconference
- March 2015: Launched project with Peking University
Contact mrct@bwh.harvard.edu for more information.
