By: Carolynn Thomas Jones
To address the need for a well-prepared, competent workforce, the National Center for Clinical and Translational Science (NCATS) has funded a grant entitled “Development, Implementation, and Assessment of Novel Training in Domain-based Competencies” (DIAMOND). This grant has four multi-PIs at University of Michigan, The Ohio State University, Tufts University and University of Rochester, and is a continuation of earlier work funded by NCATS called “Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ)” (1). These two projects have both embraced the JTF Clinical Trial Competencies, including applications of the competency domains beyond clinical trials to include social and behavioral clinical research. Individuals from the Joint Task Force were highly involved in workgroups for ECRPTQ (Stephen Sonstein, Carolynn Jones and Jonathan Seltzer, and in DIAMOND (Carolynn Jones) serves as mPI and Stephen Sonstein serves as an Advisory Board Member.
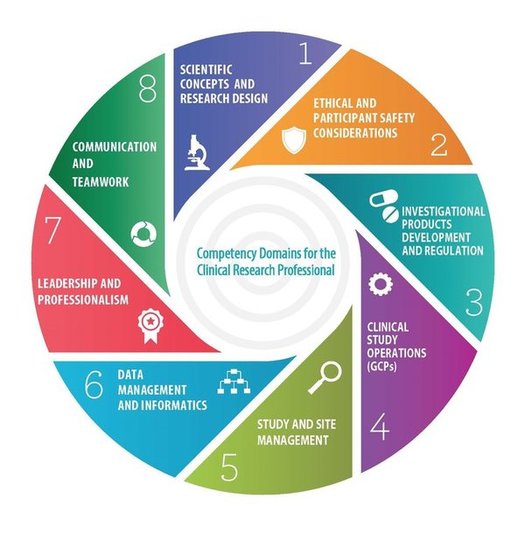 The primary aim for DIAMOND is to provide a ‘discovery learning space’ whereby individuals can share public-facing clinical research training and assessments. Initiated through Clinical and Translational Science Award (CTSA) academic medical center sites, this learning hub is publically available, not just to CTSA clinical research professionals. Users can upload training or access training freely. Notably, all training uploaded is tagged to one or more of the JTF domains, which allows for sorting of available training by domain. Users can click directly onto the JTF Wheel to select the domain that they wish to search training in. More advanced search methods are also available. This learning portal is a federated clearinghouse; therefore, all training is housed at the contributors’ servers and learners access offerings via a URL. Training is not the only linked activity. Assessments of competence are hard to locate because very little work has been done to provide assessments beyond the cognitive level. The DIAMOND Portal hopes to receive and provide access to assessments as well as learning offerings. Indeed, some of the learning offerings are coupled with assessments. Users should expect that their submitted offerings will be featured on the DIAMOND portal within 72 hours of submission.
The primary aim for DIAMOND is to provide a ‘discovery learning space’ whereby individuals can share public-facing clinical research training and assessments. Initiated through Clinical and Translational Science Award (CTSA) academic medical center sites, this learning hub is publically available, not just to CTSA clinical research professionals. Users can upload training or access training freely. Notably, all training uploaded is tagged to one or more of the JTF domains, which allows for sorting of available training by domain. Users can click directly onto the JTF Wheel to select the domain that they wish to search training in. More advanced search methods are also available. This learning portal is a federated clearinghouse; therefore, all training is housed at the contributors’ servers and learners access offerings via a URL. Training is not the only linked activity. Assessments of competence are hard to locate because very little work has been done to provide assessments beyond the cognitive level. The DIAMOND Portal hopes to receive and provide access to assessments as well as learning offerings. Indeed, some of the learning offerings are coupled with assessments. Users should expect that their submitted offerings will be featured on the DIAMOND portal within 72 hours of submission.
An additional feature of DIAMOND is the ePortfolio system that also provides an opportunity for individualized professional development planning and showcasing of knowledge, skills and abilities (KSAs) by ePortfolio users.

To access more information about DIAMOND, go to https://clic-ctsa.org/diamond
Funding for DIAMOND comes from a Collaborative Innovation Award (U01TR002013) from the CTSA program, which is administered by the NIH’s National Center for Advancing Translational Science.
References:
(1) Calvin-Naylor, N.A., Jones, C.T., Wartak, M.M., Blackwell, K., Davis, M., Divecha, R., Ellerbeck, E.F., Keiburtz, K., Koziel, M.J., Luzuriaga, K., Maddox, J., Needler, N.A., Murphy, S., Pemberton, K., Radovich, C., Rubinstein, E.P., Selker, H.P., Tenaerts, P., Unsworth, K., Wilson, K., Wright, J.E., Barohn, R., Shanley, T.P. (2017). Education and training of clinical and translational study investigators and research coordinators: a competency-based approach. Journal of Clinical and Translational Science, January 2017, pp. 1-10. DOI: 10.1017/cts.2016.2. Located at: https://doi.org/10.1017/cts.2016.2

