The MRCT Center has worked with stakeholders to develop case studies that show (1) different ways to get started returning individual research results, (2) how common roadblocks in the IRR process have been overcome, and (3) how thoughtful planning early on has enabled success with IRR.
Contact us if you would like to contribute a case study.
On May 2, 2023, the MRCT Center presented a webinar about these case studies, “Returning Individual Research Results and Data to Participants: Experience from the Field.” To watch this webinar and see related resources, click here.
Given the interest and questions that the May webinar generated, we offered a 3-part summer webinar series about the specific ethical, operational, and technical challenges to returning individual research results and data to participants.
The Digging Deeper series explored Pfizer’s Participant Data Return Solution, IRB/HRPP Responsibilities, and Returning Genetic/Genomic Secondary Findings. To view resources from these webinars, click here.
Supporting Participant Decision-Making in Genetic Testing Studies
This case study shares an example of how one institution created an educational tool to support decision-making for potential participants in a genetic testing study.
Returning Routine Lab Results to Participants During a Clinical Trial
This case study demonstrates how an industry sponsor operationalized returning routine laboratory results to participants during an ongoing, multi-site clinical trial.
Responsibly Returning Secondary Findings
This case details the experience of a research team returning secondary findings to participants in a genetic testing study.
Returning Non-Validated Test Results
This case study describes how an IRB navigated returning results of a new COVID-19 assay from a non-CLIA-certified lab during a public health emergency.
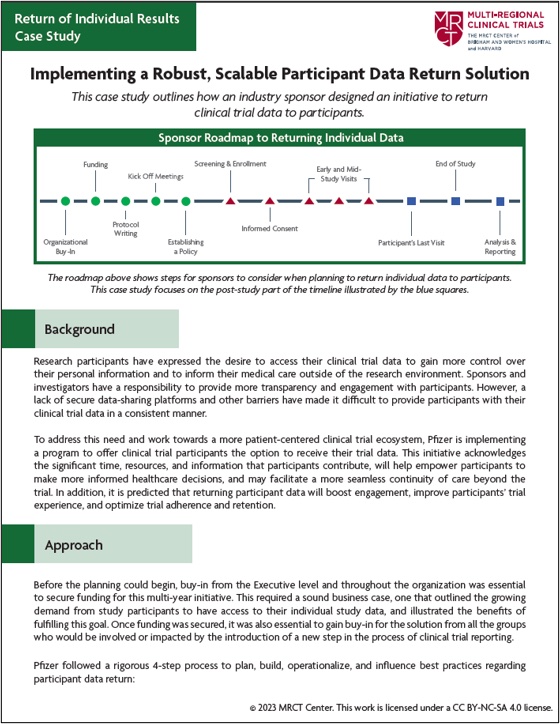
Implementing a Robust, Scalable Participant Data Return Solution
This case study outlines how an industry sponsor designed an initiative to return clinical trial data to participants.