Once you have determined what data will be generated and whether you will return the results, consider the following topics, arranged by different data types and situations below:
More specific considerations on these topics and other important points to consider can be found on the respective Results pages: Urgent, Actionable, Personally Valuable, and Have No Known Implications.
The nature of your research, the results being returned, and the resources available will help determine who should return IRR. There are various pathways to consider that involve different communicators:
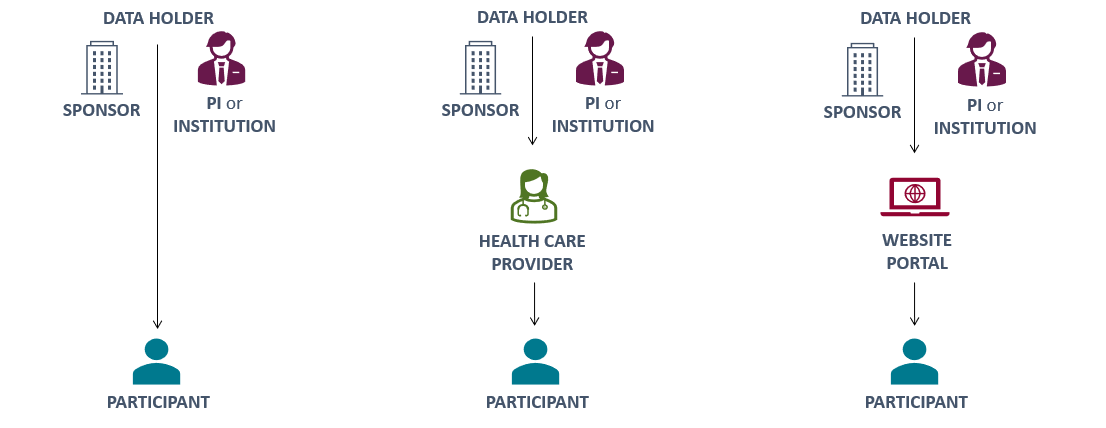
Points to consider regarding who will communicate result to participant:
PI or Institution
(or Research Site Staff):
- May require training regarding how to communicate and explain results
- Should allow for the study context to be explained with results
- Plans for who will return results should generally be addressed in study protocol, timelines, and/or contracts with sites before the study begins
- Will require current (updated) contact information
- May require additional visit
- Results may not integrate into clinical care if the tests are not performed in a certified or qualified lab
Healthcare Provider (HCP):
- May require training on how to interpret and explain results
- Research results can be communicated during medical care visits
- Conversation about results occurs with a trusted care provider
- PI needs to collect and maintain contact information for HCP
- PI should document communication of results and verify that the HCP received the information
- Note that HCP may not understand, feel comfortable with, or want responsibility for returning research results. If this is the case, consider leveraging a trusted third-party care provider
- HCP may be required to repeat the test performed if the research test did not comply with applicable regulations
- Participant may not have a HCP or health insurance
Website Portal:
- Avoids scheduling a specific appointment
- Allows data to be made available at any time, both during and after the trial as results become available
- Participant contact information is not needed once the participants have received portal instructions and created an account
- Allows for delivery of a consistent message and explanation of the result
- Written results can be supplemented with visualizations, images, and/or video
- Resource investment required to design, maintain, and secure an Internet portal
- Does not accommodate participants without internet access or technical facility
- Privacy concerns for participants, sponsors, and investigators in the event of a breach
- Participants cannot ask questions or readily get additional information
- Participant may not have or feel that they have the requisite resources/knowledge to follow up with providers on their own
- Note: Certain types of results should not be released until there is a discussion with the participant
Recommendations for who should return results of different data types:
PIs should generally give urgent results to the participant contextualized with appropriate medical information, connect with the healthcare provider, and document the handoff.
Documentation should include the result, referral, and verification of the transfer of responsibility.
It may be necessary to inform the healthcare provider first, and then inform the participant with recommended next steps. These situations include concerns around requisite psychological support systems (e.g. information indicating suicidality), or in settings wherein the nature of the problem might inhibit the ability of the participant to respond.
If the participant is unreachable in an urgent situation and no alternative person has been designated by the participant, the PI and study staff should revert to procedures deployed in clinical settings and submit a report to the IRB as soon as reasonably possible. The medically responsible person affiliated with the trial should be contacted and tasked with this responsibility whether or not that person is the principal or site investigator of the trial.
The PI should determine how to proceed in other special circumstances.
Any return pathway above may be deployed for results that are relevant to the participant’s health, are actionable, and are not urgent.
It is not generally necessary to involve a participant’s HCP in return of non-urgent results, but results should be provided in a manner where the participant can share or show them to their HCP if needed.
In some situations, it may be appropriate to connect with a participant’s HCP to confirm follow-up and/or document a transfer of responsibility.
It is not necessary to involve a participant’s HCP in cases that are not actionable. Exceptions such as results that may have a significant and/or long-term impact on the participant (e.g., certain genetic results) exist and should be addressed.
Regardless of the situation, it remains beneficial to return results in a format that is easily shared by participants with their HCPs.
Decisions for whether to and who should return this data type should be made on a case-by-case basis.
Whoever communicates these results should explain any implications and limitations of the results.
The timing of when results can be returned will vary. Depending on the result, some can be returned during screening, recruitment, during the trial, or at the participants last visit, with minimal risks to study integrity. Other results will need to be returned after the study to protect its integrity or to allow analyses to be performed.
The type and urgency of the result will determine when the result is returned. If the result is urgent, it should be returned as soon as possible.
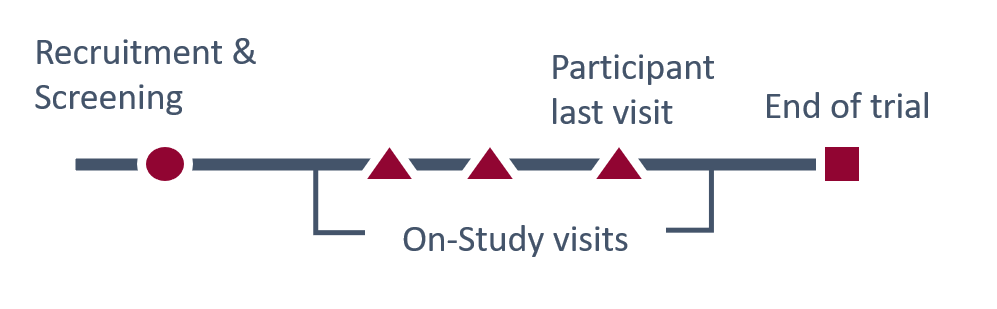
In other settings, the actionability and potential personal value of the result and its relation to study integrity and outcomes will impact whether it should be returned during the study or after the study.
Note that returning IRR after a study also provides the opportunity to provide individual results alongside aggregate results or a plain language summary. This context, in addition to being appreciated by participants, may in some cases be relevant for how a participant and/or their HCP choose to manage their care.
Urgent results or urgent incidental findings should be returned as soon as possible.
Appropriate preparation will facilitate timely return of these results; the proposed process should be included in the IRB-approved protocol.
If the process was sufficiently outlined in the protocol and an urgent situation arises, results can be returned as soon as possible without IRB consult.
If the plan that was outlined does not adequately address the situation, and if time allows without compromise to participant safety, the IRC/REC should be consulted.
If delay could risk the safety of the participant, the investigator should act on the result to minimize potential harm to the participant and inform the IRB/REC subsequently. After the report is filed, a process should be developed should a similar situation arise again
If a non-urgent result is medically actionable for the participant and will not impact study integrity, it should be returned as soon as possible. Non-urgent results related to study outcomes should generally not be returned until the study has ended or, in some cases, when the participant blind is broken.
It is logistically easier to return all results after the study has ended, so long as this timing will not impact participant safety or well-being.
A result that is personally valuable and related to study integrity or outcomes should generally not be returned until the study has ended.
Results that will not impact study integrity can be returned as soon as reasonably possible.
Sponsors should consider returning certain results of unknown significance if there is a logical reason to do so when available, when return is feasible, and with the appropriate context and information at the time.
There is no obligation to re-interpret these results for participants after the study, or to reopen old datasets as new implications are identified.
The mechanism for return will depend upon and should be considered in tandem with who is returning, what is being returned, and when. For each, consider the advantages and disadvantages, along with how the communication will be documented.
Options for return are as follows:
Two-Way Communication
Personal visit, video conference, or phone call
This communication method is often selected for urgent or sensitive results, or those that require careful discussion. This method is also often chosen for return during an ongoing clinical trial when study site resources are still available.
Points to Consider
- Provides an opportunity to ensure that the findings are presented in the proper context and explained
- Participant can ask questions
- Participant is provided with appropriate referrals for follow up medical advice and clinical care, if needed
- Investigators must generally be licensed and credentialled to provide medical advice
- Need to maintain updated contact information if communication occurs after trial has ended
- Teams should still have a plan in place in the event that a participant cannot be easily reached (potentially by capturing HCP or emergency contact information along with permission to contact in this scenario)
- May need to schedule additional visit(s) or communication
- In addition to conversation, written information can be helpful to share to avoid misinterpretation and for referral. Take-home summaries of individual results and subsequent discussion points should be provided wherever possible
- Site contracts and budgets may need to include or be amended to allow for contact
- Study plans for return of IRR should be evaluated for feasibility and compliance
One-Way Communication
Letter or portal
A static communication provides a confidential way of sharing results with participants.
Points to Consider:
- Information is consistent and accurately maintained
- Participant can refer to the written information at any time
- Participant may share their results with others (e.g., health care professionals, family)
- Portability of digital results should be considered to facilitate sharing when needed
- Avoids scheduling a specific appointment
- Decreases burden on participants
- Potential concerns for participant privacy/confidentiality if information is found or discovered unintentionally
- Participant comprehension of result difficult to assess
- Unable to address questions or assist with reaction to the information
Specific to Portals:
- Infrastructure is necessary to enable portal
- Portal utilization by participants is dependent on internet access and technical ability
Specific to Letters:
- Need to maintain up-to-date contact information
- Confirmation of receipt by participant may be difficult
- Privacy and confidentiality concerns are heightened
- May be a lower cost option for proofs of concept and/or pilots
Consider these recommendations for how to return different data types:
Urgent results should be directly communicated to the participant either via phone/video call or an in-person meeting. If these communication attempts are exhausted, the medically responsible person for the study should follow standard procedures for clinical emergencies.
The participants’ health care provider (HCP) should also be informed when appropriate. Each communication attempt with the participant and HCP should be documented, including the date, time, and method.
Decisions for how to return this data type should be made on a case-by-case basis, considering the details above.
It may be appropriate to also inform the participants’ HCP of actionable results, and study teams should plan accordingly.
Decisions for how to return this data type should be made on a case-by-case basis, considering the details above.
Decisions for how to return this data type should be made on a case-by-case basis, considering the details above.
With the exception of returning urgent results, it is important to offer participants a choice as to whether or not to receive their individual research results. In addition, you should specify whether and when participants have a right to certain information and how they may request it.
Since consent is an ongoing process, participants should be asked periodically about receiving their results as an anticipated component of voluntary and informed participation. A reliable system for storing and maintaining participants’ choices is necessary; that system should allow for changes to be documented and preserved.
Here are at least 4 choice mechanisms* for participants that depend upon the resources and plan for return. We also outline the options of not offering any choice to participants (whether that means receiving all of the results or none at all). This is strongly discouraged unless there are compelling scientific and ethical reasons for not returning results. The options are as follows:
Choice from a menu of options:
I would like to receive the following results:
______ Result A
______ Result B
______ Result C
______ I do not want to receive any of this information.
______ Please ask me again when results are available.
When is this option appropriate?
If your study will generate a myriad of results that you’d like to return and you have systems in place that would allow you to carefully track participants’ choices, you could consider allowing participants to choose which results they’d like to receive. Include a list of available results and ask participants to initial next to any results they would like to receive. Include choices that allow participants to refuse all results and/or to decide later.
Choice between all or nothing:
You may choose whether or not to receive this information.
______ Yes, I want to receive this information.
______ No, I do NOT want to receive this information.
______ Please ask me again when results are available.
When is this option appropriate?
If you have results available but will not offer a detailed choice about which results participants would like to receive, you can limit participants’ choice to receive or not receive all results (all or nothing). Ask participants to initial indicating their choice. Again, include a choice that allows participants to decide later.
Results WILL be returned and participants who do not want their results cannot participate in study:
Potential participants should know which results will be returned when deciding whether or not to participate in the study. It is helpful to explain why the only option is to receive results.
When is this option appropriate?
Generally, this option is appropriate when the study outcomes depend upon the participant receiving results (e.g., a study of behavioral interventions for weight loss).
If all results will be shared without exception, this should be emphasized during the informed consent process.
Results will NOT be returned. Participants do not have a choice, other than not participating in study:
If no results will be returned, you should explain during the consent process that results will not be returned, and why.
Potential participants should know that no results will be returned when deciding whether or not to participate in the study.
When is this option appropriate?
If no results will be shared, there should be a scientifically or ethically valid reason for not doing so. The reason should be explained in the informed consent.
Make sure to plan, explain, and include in the informed consent that urgent results will still be returned if they arise.

