HOW WE DEFINE URGENT RESULTS
An urgent result is any result that requires immediate follow-up, typically by a healthcare professional. This result could have been anticipated due to the research and condition under study, or it may be unanticipated and/or related to a previously undiagnosed condition.
Examples:
- A “routine” blood pressure found to be 220/120 mmHg
- A participant reports suicidal ideation
- A mass is discovered on a routine Chest Xray
Review the Basics for Returning Urgent Results:
How to Prepare and Additional Points to Consider
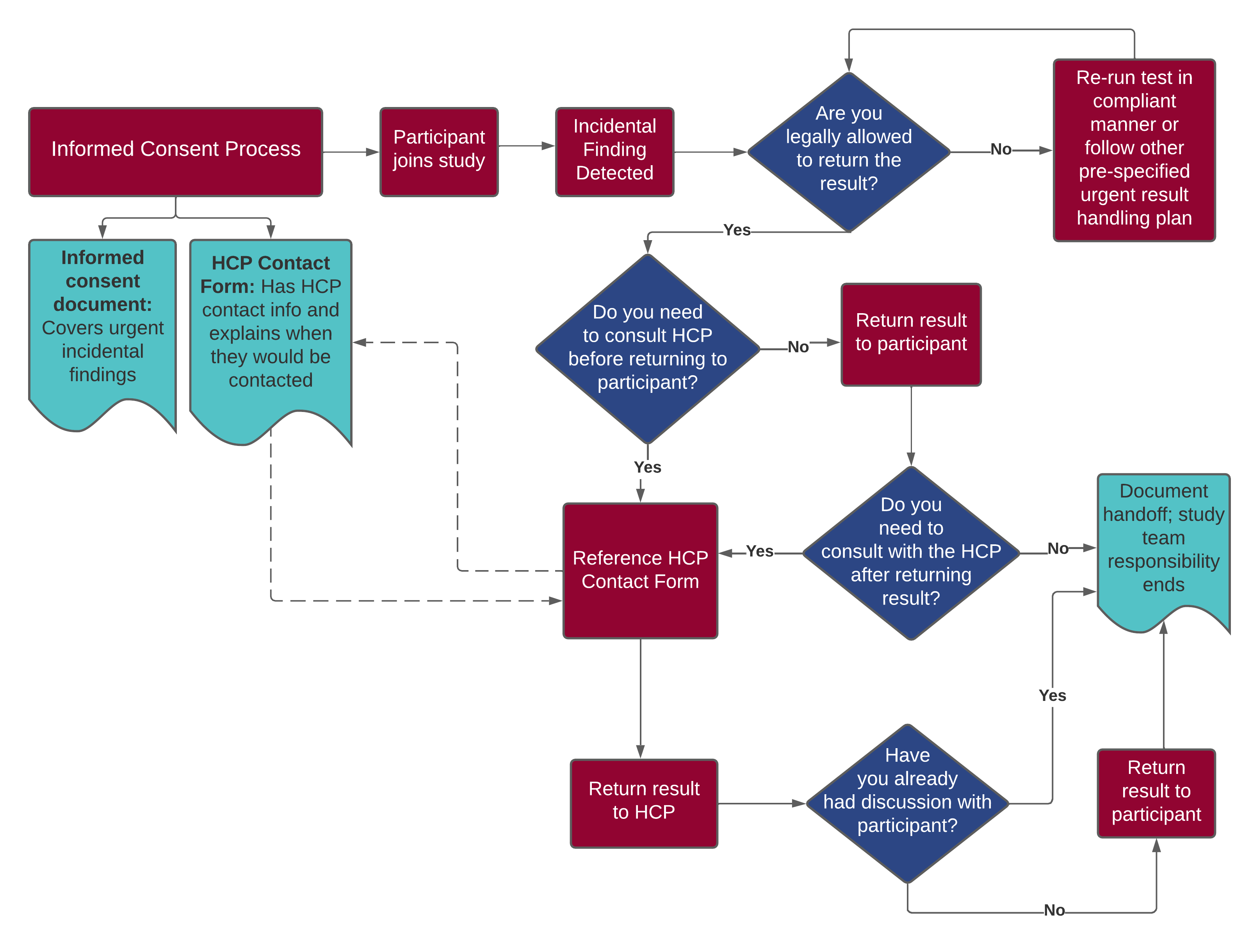
Informed Consent
The informed consent form should state that there is a possibility for the study to generate an urgent result or urgent incidental finding (hereafter “urgent result”), and that any urgent result will be returned promptly to the participant and/or their healthcare provider. In some cases, this means that the participant must consent to sharing data with the healthcare provide, even if the participant does not wish to receive other results.
Sample language:
Sample: We will [draw blood] [perform tests] that may result in medical information that requires immediate action. We will share this information with you [and your healthcare provider] so that you can get further medical care.
If you agree to take part in the study, you are agreeing to sharing this information with you [and your healthcare provider]. [We will also need your healthcare provider’s contact information]
[If you do not agree with allowing this information to be shared with your healthcare provider, you cannot participate in this study. This is for your safety.
If you do not have a healthcare provider, then…]
[If we cannot reach you to tell you about the result, please provide the name and contact information of someone you trust with whom we can share this information. If we cannot reach you after X attempts by (phone/email/text), we will contact this person.]
Healthcare provider contact information
If the involvement of the healthcare provider is necessary, the provider’s contact information should be collected as early in the study as possible, ideally during enrollment (see template here). Requesting this information can also facilitate a conversation with the research participant not only about their preferences for whom to include but also to establish realistic expectations should an urgent result arise.
Note that, along with the urgent result, any other data that might inform the healthcare provider’s action/interpretation of the urgent result should also be returned.
It should be anticipated that a potential participant without health insurance or a HCP may wish to join the study. A plan should be made for how their participation will or will not be accommodated and, if participating, how any urgent result will be handled.
This plan will vary depending on the study. For example, a PI may be asked to assist the patient to follow up locally for appropriate next steps and care. In some instances, additional funds can be made available if safety follow-up is needed
Regulatory Requirements & CLIA Confirmation
If an urgent result complies with necessary regulatory requirements for return (e.g., the test was performed in a CLIA lab), it should be returned to the participant ASAP.
If the urgent result will NOT comply with all necessary regulatory requirements for return, a plan should be made in advance for a medically responsible course of action (e.g., the study team will rerun the test in a compliant, clinically reliable manner and return it to the participant ASAP.)
See more here about regulations that pertain to IRR.
Access to appropriate expertise
Some results are challenging to interpret for actionability or validity, and/or difficult to explain to participants. A responsible medical professional, listed on the study protocol IRB application, should be identified before the study begins, to advise as needed.
If needed, referral to or provision of support (e.g., genetic counseling) should also be considered when appropriate.
Role of the IRB or Ethics Review Committee
The IRB should not be expected to interpret a medical or clinical result nor to determine actionability.
The IRB should review and decide whether the urgent finding will be considered an adverse event or unanticipated problem requiring reporting to the IRB, and if so, whether there is flexibility in timing of the report and/or other obligations to be considered.
See more here about the role of IRBs.
Process Flow