We recognize the difficulty in getting started with returning individual research results (IRR) to participants. On this page we outline steps and provide recommendations for developing policies and processes for the dissemination of individual research results (IRR).
Steps covered on this page:
Learning through experience
Developing a plan for returning IRR is often an iterative process. Build upon and improve what you already do.
Start simple, establish a process, and learn from the experience. Then pilot or implement the return of additional data types to further refine the process.
The goal is a robust program of returning results throughout an organization, a goal which will take time to achieve.
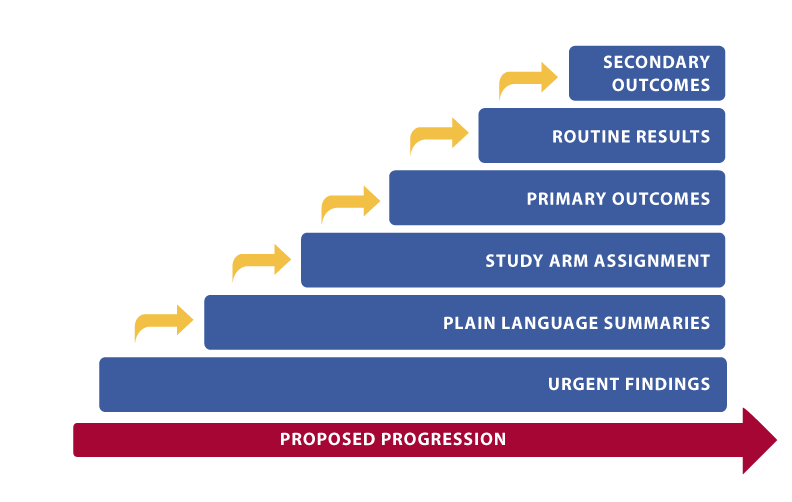
Proposed progression of return
Urgent results (during study)
You are required to return urgent, actionable findings to research participants either directly or through a healthcare provider – make sure you are prepared. Use this as an opportunity to create a robust plan during protocol development, involve key groups within your organization, and communicate clearly with participants. See more here about returning urgent results.
- Go through the process of identifying each result that will be available and the possibility for an urgent result to be generated; plan accordingly (see more here):
- Identify all possible data that will be generated by a study
- Consider incidental findings that may emerge as a consequence of the research procedures
- Out of all study data and potential incidental findings, identify data that could produce an urgent result
- Make sure those results will have analytical validity (e.g., CLIA laboratory, professional read of imaging study, consultation with medically responsible personnel), or that there is a plan for repeating test if/as needed
- Describe the specifics for your IRR plan in the protocol and documents submitted to the IRB, including the ICF (see next)
- Establish a plan for consent regarding urgent results:
- Set expectations with the study team regarding what will and will not be returned, parameters for decision, and any requisite training
- Plan to explain what will and will not be returned and the rationale during the informed consent process, and include those explanations in the ICF, making sure to emphasize that participants will not have a choice regarding the receipt of urgent results
- Decide how to obtain contact information from the participant and their healthcare provider, as needed. Click here for a template
- Establish a plan for documenting participant decisions
- Make decisions and arrangements for how to operationalize the return of an urgent result before the study begins:
- Identify medical expertise available, if necessary
- Choose method of return
- Establish how the return and/or handoff to a provider will be documented
- Practice through a table-top exercise or mock drill, soliciting feedback about the process
Plain language summaries (after study)
While communicating plain language summaries (PLS) is often executed through public dissemination of the aggregate results of a clinical trial, providing PLS directly to participants after a study has ended is a good way of preparing and establishing processes for return of IRR. See more here about returning plain language summaries.
- Establish a plan for consent for plain language summaries:
- Choose a mechanism for participants to choose whether or not to receive plain language summaries and include it in your informed consent process
- Plan to offer opportunities for participants to change their decision, and communicate those plans to participants
- Ensure through the consent process that the participant understands the role they play in keeping their contact information current and with whom they need to update with changes
- Consider logistics:
- Determine who will collect and maintain contact information for participant (and HCP, if appropriate)
- Identify how you will store and update participant choice and contact information throughout and after the study
- Establish which vendors (if any) will be involved with this task and who from your organization will manage and monitor their performance
- Ensure materials are understandable:
- Consider health literacy elements of plain language, numeracy, cultural competency, and design
- Plan for documents to be translated into additional languages as necessary, along with interpreter services as necessary when communicating with participants
- Obtain and incorporate patient/community feedback
Study arm assignment (after study)
After establishing the processes for urgent findings and PLS, other types of data return should be considered. In randomized blinded clinical trials, communicating study arm assignment at the end of the trial involves additional considerations. See more about returning actionable or personally valuable results. In addition to the steps above, consider:
- Determine whether participant HCP should be informed, and, if so, include in the informed consent document
- Assess who will communicate the result(s) to participants, and how
- Note that this may include amending contracts with sites and/or vendors
- A different communication method from urgent findings and/or plain language summaries may be appropriate or preferred, depending on the study. If that is the case, make sure that process is outlined and do another practice exercise with feedback
- Decide how participants will be able to access or receive their IRR if they withdraw or are withdrawn from the study
Primary endpoints (after study)
Advance the complexity of data return by communicating primary endpoints. This must be done after the study has ended. See more about returning actionable or personally valuable results, and consider:
- Confidentiality and privacy needs may become more complicated with more specific results. Make sure these issues are adequately addressed and planned for in study processes and site/vendor contracts
- Returning additional results may prompt more questions or conversations from/with participants – assess your communication processes (who will return results, and how), and anticipate and plan for more robust communication accordingly
Routine results (after and/or during study)
Some routine results are appropriate to return during a study, and some after a study. Consider how useful or meaningful the results will be to participants if they are received in a timely fashion vs. after the study has ended. See more about returning actionable or personally valuable results, and consider the following:
- Establish which results can be returned in real time (during the study) without compromising the integrity of the study.
- Anything related to study endpoints or that may affect participant (or investigator) blinding should be returned after the study has ended, unless there is a potential safety issue
- Again, assess your communication processes (who will return results, and how) and adjust if needed for returning new types of results and potentially new times.
- If you do adjust, ensure the process is outlined and do another practice exercise with feedback
Secondary endpoints (after study)
At this point your logistical processes should be well established for returning results. Now, your main additional consideration is communication. See more about returning actionable or personally valuable results.
- When returning secondary endpoints, communication with participants after the study has ended may need to be reconsidered and made more robust in order to explain results and answer questions. Plan processes and site contracts according to these needs
Designing a Pilot
A pilot can effectively provide a proof of concept, experience with processes and workflow, identification of problems and their correction, and confidence in returning IRR. Any pilot provides an opportunity for learning and every organization will have different approaches and challenges. Consider:
- What approvals are needed and by whom
- Which groups to involve
- Start small, balancing simplicity, flexibility, and forward progress
- Plan for iterative feedback and process for correction
- Plan the next pilot based on challenges or success of the first
- Early proof of principle is important for building momentum
- The study team will determine what data will or can be returned, when they will be returned, how they will be returned, and by whom
- Remember, simplicity is important. You will learn what works and what needs to be re-engineered. More robust tools (e.g., online portals for secure, scalable solutions) can be built in time
Who to involve in planning for return
Implementing the return of IRR will require buy-in from multiple groups. The degree to which the return of IRR is being proposed (e.g., a small pilot vs. an organizational policy) will also impact necessary buy-in.
It is important to identify the necessary reviews and approvals that are needed before the return of IRR is initiated. Working in partnership early will ensure that concerns and needs are addressed and contribute to success. It may be especially helpful to identify a “champion” within each group. Of course, the specific groups will vary by organization and role (e.g., industry vs. research site vs. investigator) as will the nature of the issues. Consider:
Groups to Engage:
- Study Team & Investigator
- General Counsel (legal)
- Privacy Office
- IT/Data management
- Human Research Protection Program Office/Institutional Review Board (IRB)
- Ethics board or Bioethics Council
- Regulatory Affairs/Country Experts
- Clinical Operations
- External vendors
- Biorepository
- Patient and Family Advisory Board
- Community representation
Potential Issues to Address:
- Regulatory compliance (local, state, country)
- Informed consent considerations
- Liability
- Study Integrity
- Identifying any necessary expertise relating to the results that will be generated/returned
To address why returning IRR is important, see more here on why to get started.
Additionally, we have developed roadmaps that outline different stakeholders’ roles within IRR for: Sponsors/Industry, PIs/Sites/Study Staff, IRBs/HRPPs, and Participants.
Methods for returning individual research results
When getting started, key decisions will need to be made around how to operationalize the return of IRR. These methods may change over time as programs and technologies evolve. When deciding which methods to use, consider the nature of your results and the resources of your organization.
Key decisions to make:
- What to return
- Who will return it
- How it will be returned
- When it will be returned
- The mechanism for a participant to choose whether or not to receive IRR
Depending on what you decide to return, each data type will have additional specific considerations. Learn more about returning results that are:
Establishing a policy
In time, the organization will determine whether and how to scale the practice of returning individual results to participants. It is helpful to develop standard operating procedures that can be changed as necessary whenever experience suggests adaptation would be helpful. Organizational guidance has the advantage of being a reference document without suggesting a mandate or requirement. At some point, however, it will be helpful to have an organizational policy. We include a model policy as an example and starting point, anticipating that every organization will adapt the model to its specific situation (link, when it exists).
SOPs, guidance, and policies can be developed over time. While SOPs will address the “who, when, how, and what” of returning results, it is also helpful to address anticipated complex situations such as participant data requests that are outside the scope of what was intended to be returned, incidental findings that are medically actionable and must be returned, and healthcare provider or family member requests for information. The availability of guidance will result in greater efficiency, less opportunity for error and liability, and an improved participant experience.
In general, the SOPs, guidance, and policy should address:
- How to approach planning for data return during protocol development
- Identifying all data and types of results that will be generated during the study
- Of those data, deciding which to return. Organizations may have different approaches to categorizing data to be returned.
- Deciding and planning for who will return the data and in what timeframe
- Ensuring necessary funding is available to implement the data return
- Study-specific documentation required
- Steps/processes to follow to return those data
- Timelines and process flows to follow
- Communicating with sites and/or vendors about their role
- Communicating with IRB/HRPP/Ethics board about their review requirements and their criteria for returning IRR
- Communicating with IRB, regulatory, and/or legal to understand country specific requirements
- Consultation with content experts for more complex data return (e.g. genetic counselors and/or those with genomic research expertise)
- All internal sign-off needed for feasibility and compliance
- ICF prototype language
- Consultation with health literacy experts or others who can aid in ensuring results will be understood by participants
- The team should be alert to additional issues that arise during and after a pilot or program expansion, and annotate the SOPs and guidance as appropriate

