Step 1: During the study, when are you collecting data?
Consider the entire clinical trial lifecycle for a participant:
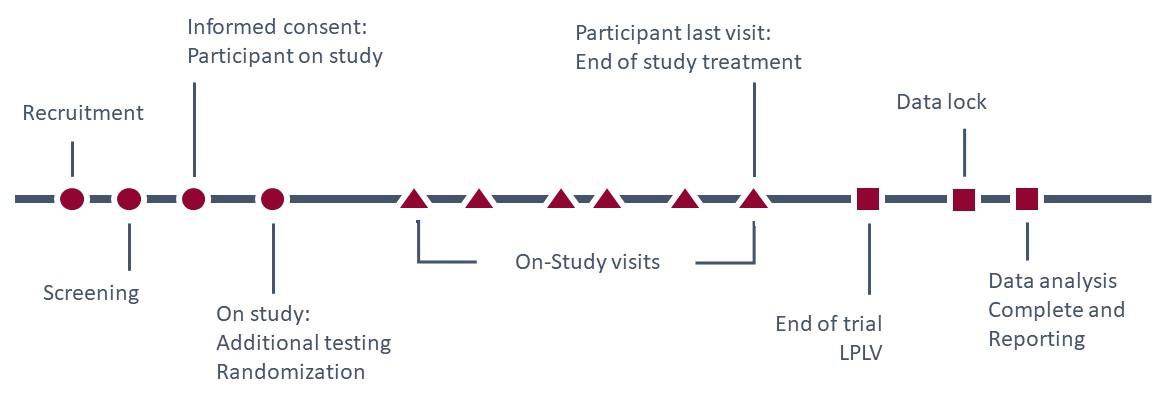
Step 2: What kinds of data will be generated during each period?
Think about the kinds of tests you will perform during each of the above periods:
- Study arm assignment
- Laboratory tests
- Diagnostic Imaging
- Genetic sequencing
- Physical or visual examinations
- Socio-behavioral
- Surveys and questionnaires
- Digital or mobile health results
- Patient-reported outcomes (PROs)
- Exploratory results
Step 3: Consider the following questions/domains for each data type during each study period to determine whether to return and what plans must be made to return:
A. URGENCY
- Is there a possibility for a result to require urgent medical attention?
- Example: A “routine” blood pressure found to be 220/120 mmHg
- Example: A participant reports suicidal ideation
- Is there a possibility for an urgent unanticipated finding to be identified while testing for something else?
- Example: A mass is discovered on a routine Chest X-ray
B. ACTIONABILITY
Does the result have established health or other personal implications and is actionable, but not urgent?
- Example: A Hemoglobin AIC (or HbA1c) blood test, a measure of average blood sugar, is above normal, and may indicate diabetes or pre-diabetes.
- Example: Genetic screening of an individual who has been personally unaffected by cancer returns the presence of BRCA1, a breast cancer susceptibility gene.
C. PERSONAL VALUE
Is the result something that could be considered meaningful or valuable to the participant despite not being currently actionable?
- Example: Genetic screening of a normal individual returns cystic fibrosis carrier status.
- Example: The participant is found to have Gilbert’s syndrome with an abnormal bilirubin secondary to a variant of a gene but one that does not cause liver disease. It is important to know, however, so that unnecessary tests are not performed.
- Example: The participant is found to have a rare blood type.
D. INTERPRETATION LIMITATIONS
Is the result not known to have any health implications?
- Example: A minimally high-density lipoprotein (HDL) of uncertain significance.
- Example: Initial genetic research studies that are trying to uncover associations between differences in our DNA and response to an investigational drug.

